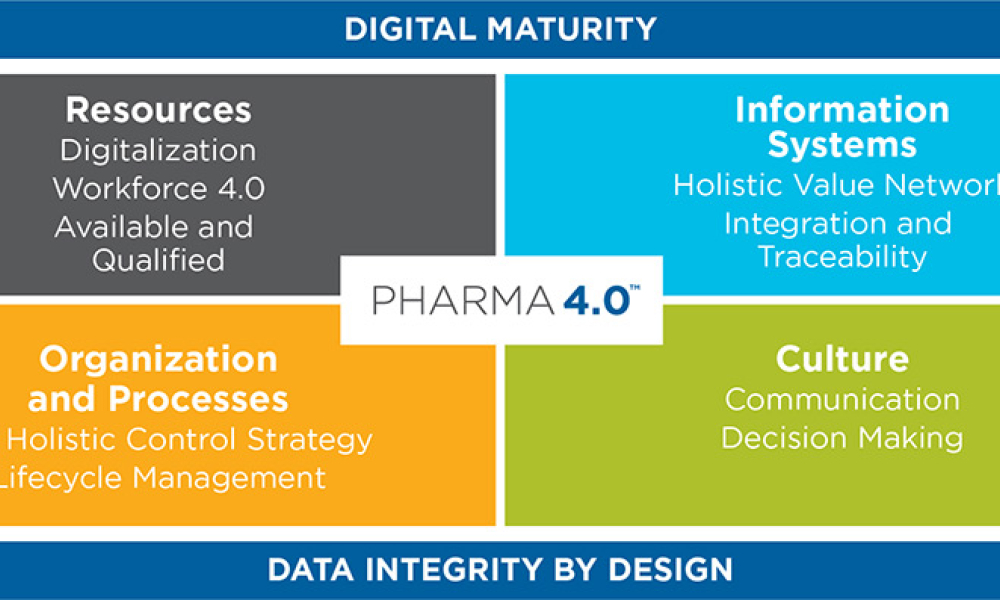
ISPE and its members are developing the roadmap to introduce Industry 4.0, also referred to as the Smart Factory, to the pharmaceutical industry as Pharma 4.0™.
Mission Statement
The aim is to provide practical guidance, embedding regulatory best practices, to accelerate Pharma 4.0™ transformations. The objective is to enable organizations involved in the pharmaceutical product lifecycle to leverage the full potential of digitalization to provide faster therapeutic innovations and improved production processes for the benefit of patients.

Implementing new Industry 4.0-based manufacturing concepts in Pharma 4.0™ requires alignment of expectations, definitions, and interpretation, with global pharmaceutical regulations.
While Industry 4.0 has been called a new industrial revolution, Pharma 4.0™ implementation will more likely resemble an evolution in which digitalization and automation meet very complex product portfolios and cycles. It is therefore important to achieve an accepted understanding of readiness and maturity, starting with additional digital enablers and elements added to the ICH Q10: The Pharmaceutical Quality System along the product life cycle. It is also important to develop business cases to showcase which Industry 4.0 automation and digitalization technologies can be applied to the pharmaceutical industry and what implications are faced due to the increasingly complex regulatory challenges in the pharmaceutical and biotechnology industries.
Digitalization, an important component of Pharma 4.0™, will connect everything, creating new levels of transparency and adaptivity for a “smart” plant floor. This will enable faster decision-making, and provide in-line and on-time control over business, operations, quality, and regulatory compliance. Notably, this new connectedness will require higher levels of security, since linked systems heighten vulnerability.
Community of Practice
The ISPE Pharma 4.0™ Community of Practice (CoP) is comprised of ISPE Members engaged in implementing Pharma 4.0™ principles in their organizations or simply wanting to learn more about the concepts and network with other members interested in the topic. Membership in the Pharma 4.0™ CoP is open to all ISPE Members. For information on joining, visit the ISPE Communities of Practice page.
ISPE Pharma 4.0 Leadership Team
Steering Committee Leaders
Sub-Committee Leaders

From Industry 4.0 to Pharma 4.0™ Operating Model
ISPE’s Pharma 4.0™ CoP Steering Committee and Working Groups have developed an operating model to apply the principles of Industry 4.0 to Pharma 4.0™ which is summarized in the image below.
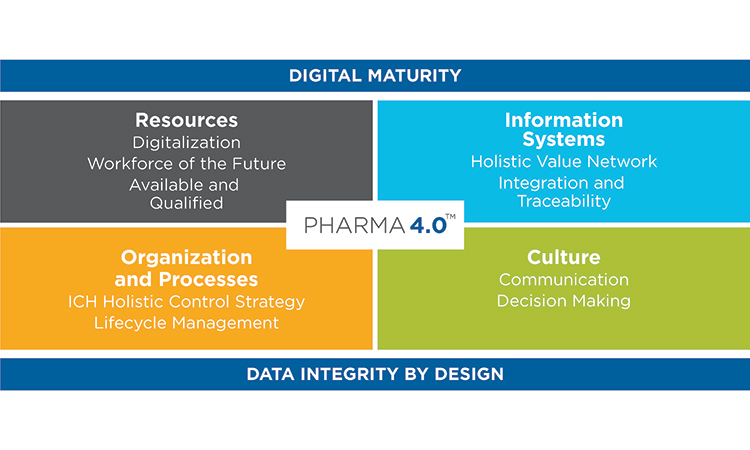
From Industry 4.0 to Pharma 4.0™ Operating Model Working Groups
- Holistic Digital Enablement
- How to provide holistic digital solutions to enable consistent, predictable, mature implementation of Pharma 4.0
- Leads: Nuha al Hafez, Roche and Yvonne Duckworth, CRB
- Process Maps and Critical Thinking
- How to develop process and data mapping to virtual models
- Leads: Volker Roeder, Arcondis and Emmie Heeren
- Plug and Produce
- Enable the transformation from centralized systems to modular, distributed, autonomous manufacturing services
- Leads: Wolfgang Winter, Agilent and Josef Trapl, Memo3 GmbH
- , Memo3 GmbHValidation 4.0
- The new paradigm of a less complex validation model
- Leads: Michelle Vuolo, Tulip and David Margetts, Factory Talk
- Management Communication Strategy
- The Pharma 4.0™ elevator pitch for management
- Leads: Teresa Minero, LifeBee and Davide Smaldone, Kenvue
- Continuous Process Verification & Process Automation
- The way to parametric release
- Leads: Alicia Tébar, QBD Consulting and Miquel Romero, Almirall
Bridging the ISPE Pharma 4.0™ Operating Model with the CoP Working Groups
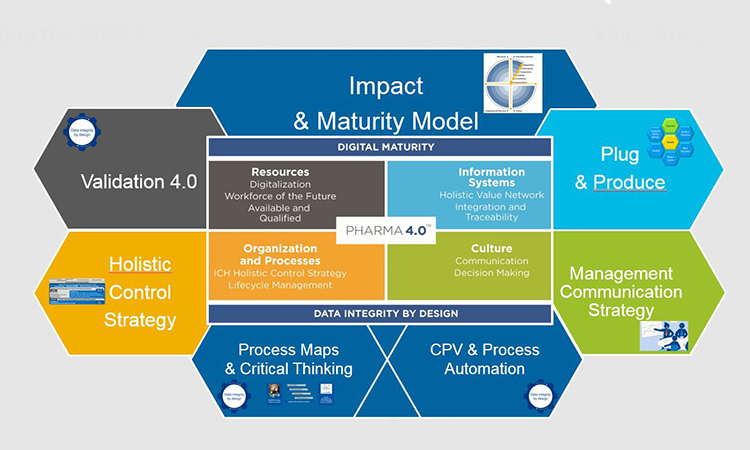
The 12 Theses for Pharma 4.0™
- Pharma 4.0™ extends/describes the Industry 4.0 Operating Model for medicinal products
- In difference to common Industry 4.0 approaches, Pharma 4.0™ embeds health regulations best practices.
- Pharma 4.0™ breaks silos in organizations by building bridges between industry, regulators and healthcare and all other stakeholders.
- For the next Generation Medicinal Products, Pharma 4.0™ is THE enabler and business case.
- For the established products, Pharma 4.0™ offers new business cases
- Investment calculations for Pharma 4.0™ require innovative approaches for business case calculations.
- Prerequisite for Pharma 4.0™ is an established PQS and controlled processes & products.
- Pharma 4.0™ is not an IT Project.
- The Pharma 4.0™ Operating Model incorporates next to IT also the organizational, cultural, processes & resources aspects.
- The Pharma 4.0™ Maturity Model allows aligning the organizations operating model for innovative and established industries, suppliers and contractors to an appropriate desired state.
- Pharma 4.0™ is not a must, but a competitive advantage. Missing Pharma 4.0™ might be a business risk.
- When moving from blockbusters to niche products and personalized medicines, Pharma 4.0™ offers new ways to look at business cases.
Pharma 4.0™ Publications
-
Technical
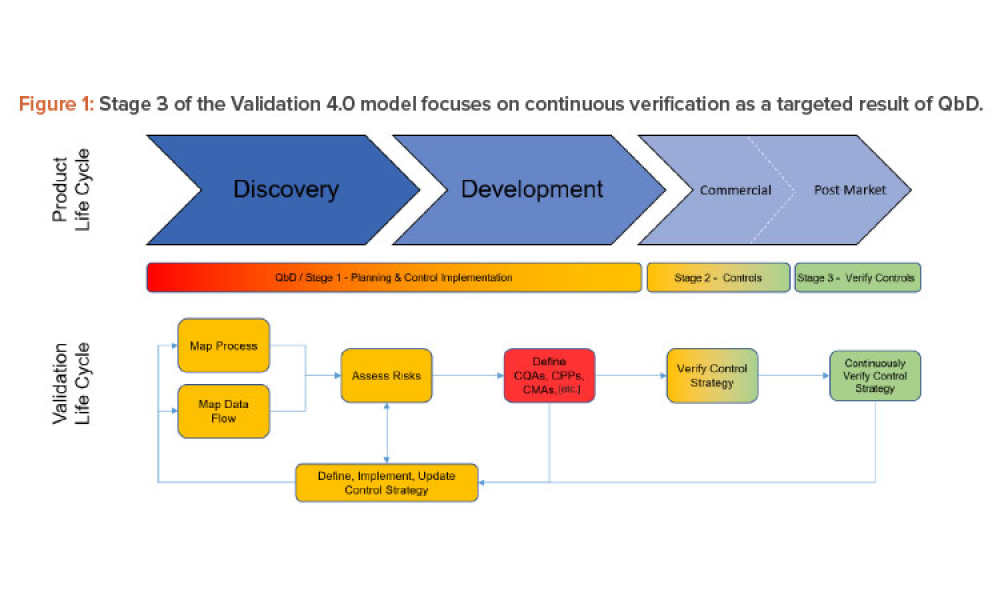
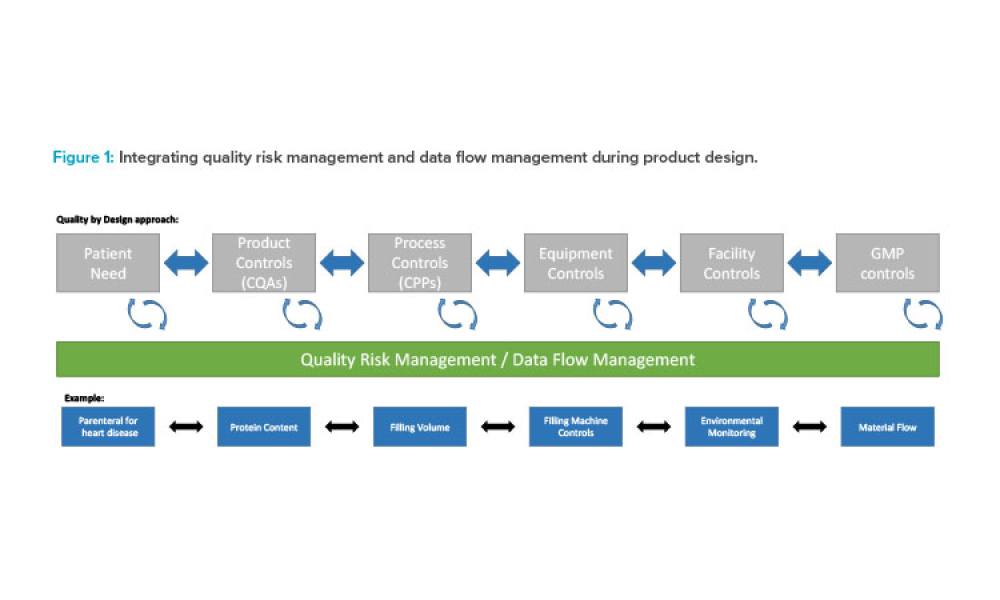
Three case studies on Validation 4.0 demonstrate how quality by design (QbD) principles, when applied with digitization, can verify processes in scale-up and technology transfer, and why blend and content uniformity matter for tablet integrity.
-
iSpeak Blog
ISPE’s Pharma 4.0™ initiative provides guidance, aligned with the regulatory requirements specific to the pharmaceutical industry, to accelerate Pharma 4.0™ transformations. Also known as the Smart Factory, the objective of Pharma 4.0 is to enable organizations involved in the product lifecycle to leverage the full potential of digitalization to provide faster innovations for the benefit of...
-
Features
-
Features
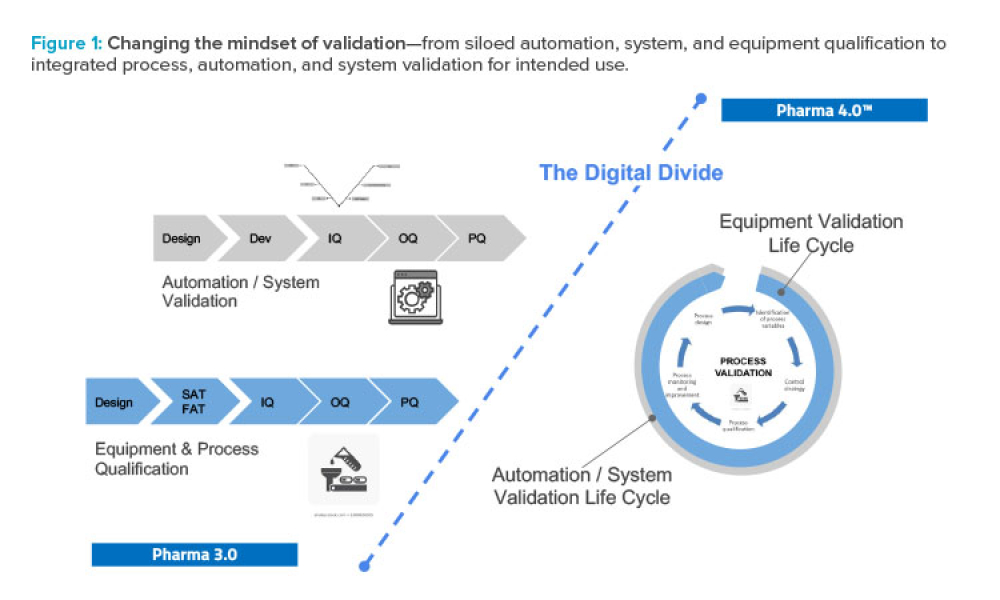
Across every industry today, digitalization is driving the use and value of data to disrupt traditional business models and ways of working. In pharmaceuticals, the promises of Industry 4.0 are expected, and needed, to finally modernize the legacy approaches that have evolved since the 1970s. Validation is an obvious target for digital disruption because of the inefficient, document-heavy...
-
iSpeak Blog
The Life Sciences industries adhere to strict documentation practices to prove quality and compliance. We’re all familiar with the truism “If it isn’t written down, it didn’t happen.”
-
Features
As Pharma 4.0™ increasingly becomes reality, our validation practices must change. We can no longer apply 20th-century thinking to 21st-century technology and resources. Validation must adapt to industry shifts from iterative to...
-
Features
As the old saying goes, “Time is money.” In today’s industrialized world, this adage is profoundly true. Manufacturers can no longer afford to overlook operational excellence. A new production philosophy called “Lean manufacturing” has been developed to save as much time as possible during manufacturing processes. In some industries, such as the automotive sector, Lean has almost been...
-
InTouch
The 2019 ISPE Europe Pharma 4.0™ Conference held in Manchester, UK, on 20–21 November 2019 was attended by 300 participants, all of whom contributed to the growing momentum for
-
Features
Applying emerging technologies can lead to more robust and flexible manufacturing processes that in turn can help the pharmaceutical industry respond to drug shortages, reduce interruptions in production and delivery of medicines, ensure consistent clinical performance of products, and achieve other benefits. Although some may believe that regulators are averse to the use of emerging...
-
Features
Every important cause needs its champion. Champions have a vision of how things should be, and a passion to reach their goals. They are committed and determined to achieve positive results, are willing to do the heavy lifting, and will take consistent and massive action until results are achieved.
-
Special Reports
More than 800 attendees met in Dublin, Ireland, on 1–4 April for the 2019 ISPE Europe Annual Conference—a new record attendance for this conference! Participants at ISPE’s sixth Europe conference learned about...
-
Features
The Pharma 4.0™ Special Interest Group is focusing on key technologies that will modernize pharmaceutical manufacturing and facilitate digital transformation. These technologies include digital twins, augmented reality, artificial...
-
-
Features
Innovative technologies such as continuous manufacturing (CM) bring speed, efficiency, and agility to pharmaceutical manufacturing together with enhanced process robustness and assurance of product quality. During CM, material is simultaneously charged and discharged into process unit operations. Similar to batch manufacturing, CM requires a comprehensive and holistic control strategy...
-
Special Reports
As members of the Architecture Team of the ISPE Pharma 4.0™ Plug & Produce Initiative, we would like to share the principles we have developed with the broader life sciences community. Our aim is to foster further exchange and...
View More Publications
Must be an ISPE member to view locked content.
Podcast: Pharma 4.0™
Listen in as Barbara Peck, ISPE, and Christian Wölbeling, Senior Director Global Accounts, Werum IT Solutions, discuss the history of Pharma 4.0™, what it is, and how it can benefit your organization.
Developed in 2017, Pharma 4.0™ will help pharmaceutical companies create a fully automated environment that considers data integrity from the beginning of the design period. Christian also reflects on the future of the new digital revolution and explains how you can get involved now.
Conference
2022 ISPE Pharma 4.0™ & Annex 1 Conference
7 - 8 December 2022
Vienna, Austria and Virtual