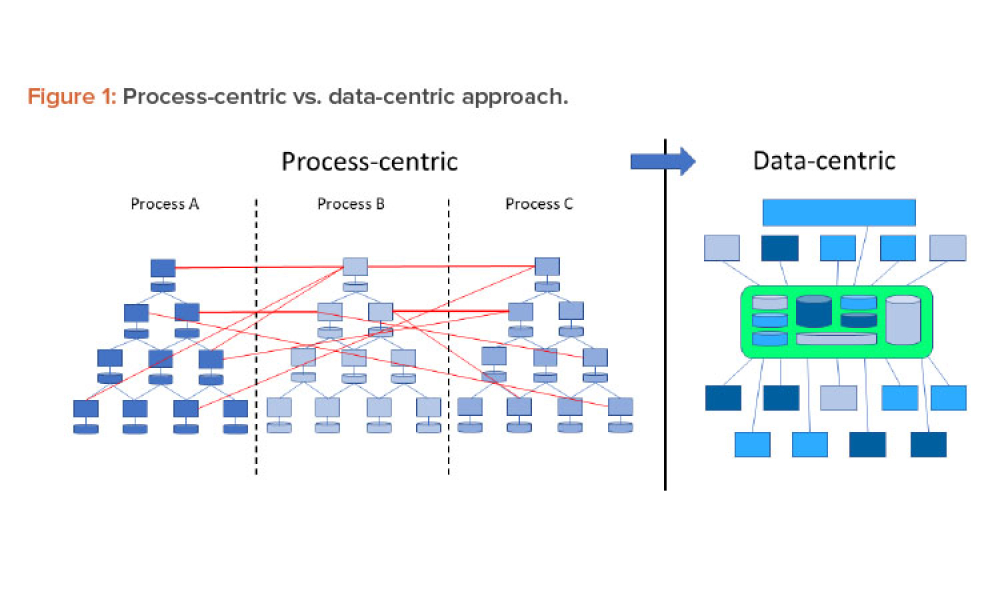
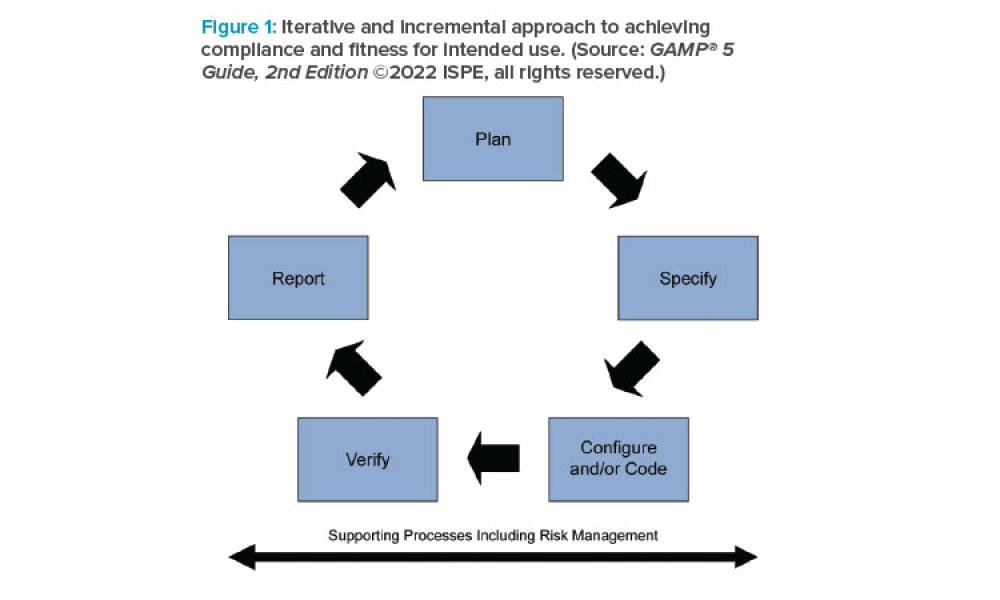
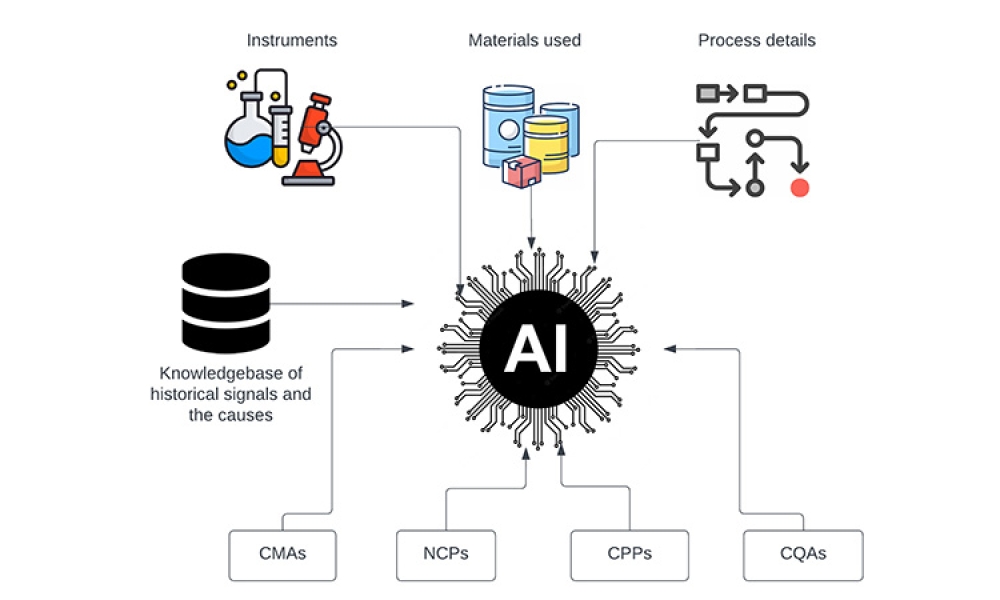
Content focuses on the financial impact of data management systems on drug development, manufacturing, and distribution; the basic computer system life cycle model and the activities and software quality assurance practices in each phase; and the controls and methods necessary to maintain data integrity and security.
Related Guidance Documents
Advanced Manufacturing (1)
Commissioning & Qualification (2)
Data Integrity (15)
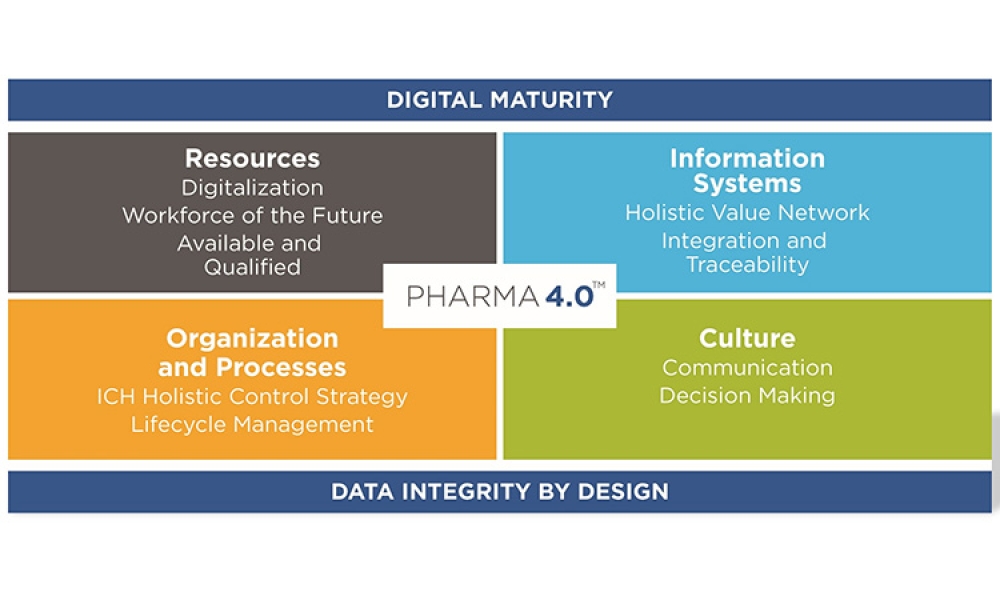
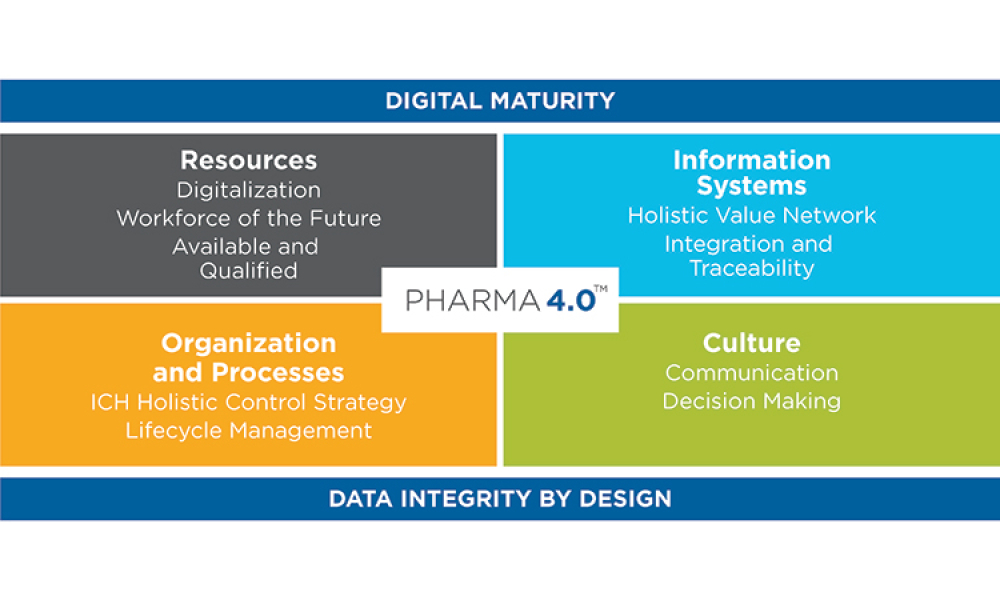
- Baseline Guide Vol 8: Pharma 4.0 1st Edition
- GAMP Good Practice Guide: GxP Process Control Systems 2nd Edition
- GAMP Guide: Records & Data Integrity Download - USD
- GAMP Good Practice Guide: Global Info Systems Control & Compliance 2nd Edition
- GAMP RDI Good Practice Guide: Data Integrity - Key Concepts
- ISPE GAMP RDI Good Practice GAMP Good Practice Guide: Data Integrity - Manufacturing Records
- GAMP Good Practice Guide: Calibration Management 2nd Edition
- GAMP RDI Good Practice Guide: Data Integrity by Design
- GAMP Good Practice Guide: Operation of GxP Computerized Systems
- GAMP 5 (2nd Ed) Download - USD
- GAMP Good Practice Guide: Enabling Innovation.
- GAMP Good Practice Guide: Testing GxP Systems 2nd Edition
- GAMP Good Practice Guide: Regulated Mobile Applications
- GAMP Good Practice Guide: IT Infrastructure Control & Compliance 2nd Edition
- GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition
GAMP® (15)
- GAMP Good Practice Guide: GxP Process Control Systems 2nd Edition
- GAMP Guide: Records & Data Integrity Download - USD
- GAMP Good Practice Guide: Global Info Systems Control & Compliance 2nd Edition
- GAMP RDI Good Practice Guide: Data Integrity - Key Concepts
- ISPE GAMP RDI Good Practice GAMP Good Practice Guide: Data Integrity - Manufacturing Records
- GAMP Good Practice Guide: Calibration Management 2nd Edition
- GAMP RDI Good Practice Guide: Data Integrity by Design
- GAMP Good Practice Guide: Operation of GxP Computerized Systems
- GAMP 5 (2nd Ed) Download - USD
- GAMP Good Practice Guide: Enabling Innovation.
- GAMP Good Practice Guide: Testing GxP Systems 2nd Edition
- GAMP Good Practice Guide: Regulated Mobile Applications
- GAMP Good Practice Guide: Computerized GCP Systems & Data
- GAMP Good Practice Guide: IT Infrastructure Control & Compliance 2nd Edition
- GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition
Knowledge Management (1)
Pharma 4.0™ (1)
Validation (12)
- GAMP Good Practice Guide: GxP Process Control Systems 2nd Edition
- GAMP Guide: Records & Data Integrity Download - USD
- GAMP RDI Good Practice Guide: Data Integrity - Key Concepts
- ISPE GAMP RDI Good Practice GAMP Good Practice Guide: Data Integrity - Manufacturing Records
- GAMP Good Practice Guide: Calibration Management 2nd Edition
- GAMP Good Practice Guide: Operation of GxP Computerized Systems
- GAMP 5 (2nd Ed) Download - USD
- GAMP Good Practice Guide: Enabling Innovation.
- GAMP Good Practice Guide: Regulated Mobile Applications
- GAMP Good Practice Guide: Computerized GCP Systems & Data
- GAMP Good Practice Guide: IT Infrastructure Control & Compliance 2nd Edition
- GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition
Community Discussions
Community Discussions
Nov 04, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Oct 30, 2024
Pharmaceutical Engineering Magazine Articles
Webinars Related to Information Systems
Concept and Discussion Papers
Process Events for the Life Science Industry Information Model Concept on OPC UA for Alarms and Audit Trails
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
A Simplified Integration of Qualified Laboratory Devices with the Asset Administration Shell as the Digital Twin
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…
Bio-Fluorescent Particle Counter (BFPC) Continuous Environmental Viable Particle Monitoring Strategy for Aseptic Filling
1 Introduction Aseptic filling has undergone tremendous development in the past decades, from manned…
March / April 2022
This article aims to refresh information on open-source software (OSS) within regulated computerized…

iSpeak Blog Posts Related to Information Systems
Training Courses Related to Information System
GAMP® Data Integrity 21 CFR Part 11, 2-Day Training Course
The importance and amount of data being generated to ensure product quality and patient safety continues to grow, and proper controls around that data continue to be a subject of regulatory scrutiny. Regulatory agencies across the world are repeatedly citing data integrity issues. As a result, industry guidance and enforcement strategies are evolving. Regulatory concerns and warning letters have extended to all areas of the pharmaceutical, biotech, and medical device business, including manufacturing, development, clinical, pharmacovigilance and other areas of the product lifecycle. This…
GMP Fundamentals: Quality System
To ensure pharmaceuticals meet specifications and regulatory requirements, manufacturers must create, implement, and follow quality systems. A “Quality System” is blueprint for a pharmaceutical manufacturer. It outlines quality philosophies and processes, provides details about how people work together, and establishes performance specifications. The Quality System provides mechanisms to monitor and control processes, systems, and people to ensure the highest level of safety, quality, and efficacy. In this On Demand course (part 3 of 11), you will learn the elements of a Quality System, as…
Computerized Systems Inspections in the Pharmaceutical Industry
This course, the third in a four-part series, has been designed by ISPE and UL EduNeering, in cooperation with the Food and Drug Administration (FDA)/Office of Regulatory Affairs ORA, to assist FDA personnel in recognizing the critical aspects of computerized systems in the pharmaceutical industry during Pre-Approval and routine current Good Manufacturing Practices cGMP inspections. The course explains how computerized systems are used in the pharmaceutical manufacturing process and provides an approach to inspecting these systems.
Cloud Concepts - Cyber Security and Block Chain
This online course will explore the pros and cons of cloud-based computing. Attendees will gain an understanding of what "the cloud" is and how it's both different, and similar, in comparison to software solutions that run on company-owned servers. Data concerns will also be addressed and aspects of regulatory compliance will be explored and answers on how one can comply will be provided. If already operating in the cloud, attendees will be gain insight on how they can assess their own company's cloud platform, and take proactive measures to resolve any vulnerabilities.
Asset Information Lifecycle Modelling
The information that forms the records of an operating facility are increasingly being recognized as a vital part of a facility. This ' digital asset ' reflects the value of data that mirrors the physical assets. The genesis of this digital asset (engineering data) is created and collected from various disparate sources. This information is also consumed by a variety of disparate functions. To facilitate the efficient creation, manipulation and consumption of engineering information it is important to define a master data model for the whole asset lifecycle . The identification of the…
Basic Principles of Computerized Systems Compliance
Basic Principles of Computerized Systems Compliance: Applying the GAMP5® Guide: A Risk-Based Approach to Compliant GxP Computerized Systems This fundamental online course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and explores tried, tested, and internationally recognized methods of meeting those requirements. GAMP guidance provides a pragmatic and effective framework for achieving computerized systems that are fit for intended use and meet current regulatory requirements, by building upon existing industry good practice in an…
View Course
Quality Management Systems Training Course
Through lecture and group exercises this course illustrates how quality systems work, the purpose of the different elements, how they connect to each other and how to recognize and transfer knowledge/connectivity throughout the organization. The diagram below from ICH Q10, covers the product life cycle for a PQS/QMS system and all aspects will be covered by this course.
Featured Conferences

Expert Xchanges
Upcoming
None Available
Past
ISPE Expert Xchange GAMP®: Realizing the Value in Validation with Critical Thinking and Computer Software Assurance
Session Length:
4 hours
ISPE Expert Xchange: Enabling Pharma 4.0™ through Plug & Produce
Session Length:
3 hours 30 min