Data Integrity (DI) is part of the mission to ensure the safety, efficacy, and quality of products produced by the pharmaceutical industry, the global regulators (e.g., the US FDA, EMA, etc.) expect that all data submitted by manufacturers to obtain market approval is both reliable and accurate. Regulators consider the integrity of data, from the moment it is generated to the end of its life cycle, to be a critical component of ensuring that only high-quality and safe drugs are manufactured. Data integrity requires that all data be attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available.
Related Guidance Documents
Advanced Manufacturing (1)
Commissioning & Qualification (1)
Data Integrity (16)
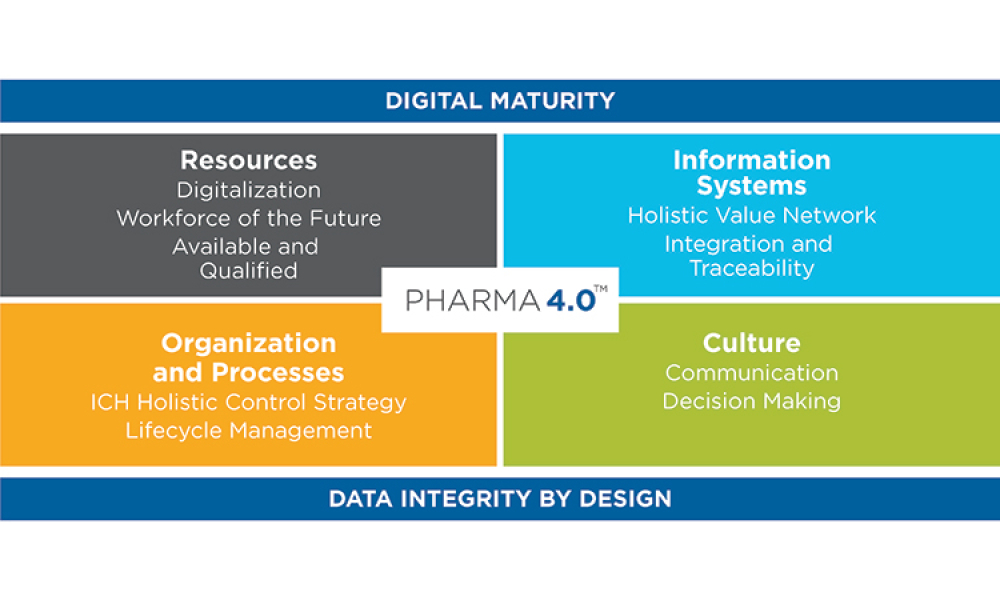
- Baseline Guide Vol 8: Pharma 4.0 1st Edition
- GAMP Good Practice Guide: GxP Process Control Systems 2nd Edition
- GAMP Guide: Records & Data Integrity Download - USD
- GAMP Good Practice Guide: Global Info Systems Control & Compliance 2nd Edition
- GAMP Good Practice Guide: Manufacturing Execution Systems
- GAMP RDI Good Practice Guide: Data Integrity - Key Concepts
- ISPE GAMP RDI Good Practice GAMP Good Practice Guide: Data Integrity - Manufacturing Records
- GAMP Good Practice Guide: Calibration Management 2nd Edition
- GAMP RDI Good Practice Guide: Data Integrity by Design
- GAMP Good Practice Guide: Operation of GxP Computerized Systems
- GAMP 5 (2nd Ed) Download - USD
- GAMP Good Practice Guide: Enabling Innovation.
- GAMP Good Practice Guide: Testing GxP Systems 2nd Edition
- GAMP Good Practice Guide: Regulated Mobile Applications
- GAMP Good Practice Guide: IT Infrastructure Control & Compliance 2nd Edition
- GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition
GAMP® (15)
- GAMP Good Practice Guide: GxP Process Control Systems 2nd Edition
- GAMP Guide: Records & Data Integrity Download - USD
- GAMP Good Practice Guide: Global Info Systems Control & Compliance 2nd Edition
- GAMP Good Practice Guide: Manufacturing Execution Systems
- GAMP RDI Good Practice Guide: Data Integrity - Key Concepts
- ISPE GAMP RDI Good Practice GAMP Good Practice Guide: Data Integrity - Manufacturing Records
- GAMP Good Practice Guide: Calibration Management 2nd Edition
- GAMP RDI Good Practice Guide: Data Integrity by Design
- GAMP Good Practice Guide: Operation of GxP Computerized Systems
- GAMP 5 (2nd Ed) Download - USD
- GAMP Good Practice Guide: Enabling Innovation.
- GAMP Good Practice Guide: Testing GxP Systems 2nd Edition
- GAMP Good Practice Guide: Regulated Mobile Applications
- GAMP Good Practice Guide: IT Infrastructure Control & Compliance 2nd Edition
- GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition
Knowledge Management (1)
Manufacturing Operations (1)
Pharma 4.0™ (1)
Validation (11)
- GAMP Good Practice Guide: GxP Process Control Systems 2nd Edition
- GAMP Guide: Records & Data Integrity Download - USD
- GAMP RDI Good Practice Guide: Data Integrity - Key Concepts
- ISPE GAMP RDI Good Practice GAMP Good Practice Guide: Data Integrity - Manufacturing Records
- GAMP Good Practice Guide: Calibration Management 2nd Edition
- GAMP Good Practice Guide: Operation of GxP Computerized Systems
- GAMP 5 (2nd Ed) Download - USD
- GAMP Good Practice Guide: Enabling Innovation.
- GAMP Good Practice Guide: Regulated Mobile Applications
- GAMP Good Practice Guide: IT Infrastructure Control & Compliance 2nd Edition
- GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition
Community Discussions
Community Discussions
Nov 04, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Oct 30, 2024
Webinars Related to Data Integrity
iSpeak Blog Posts Related to Data Integrity
Pharmaceutical Engineering Magazine Articles
Videos Related to Data Integrity
Concept and Discussion Papers
Process Events for the Life Science Industry Information Model Concept on OPC UA for Alarms and Audit Trails
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
Using SaaS in a Regulated Environment – A Life Cycle Approach to Risk Management
1 Introduction In the evolving regulated IT environment there are many things to consider when…
Data Privacy: A Compliance Blind Spot
1 Introduction What should be planned for when implementing a new computerized system, such as a…
Considerations for a Corporate Data Integrity Program
Data integrity is a global expectation as evidenced by the Medicines and Healthcare Products…

Pharmaceutical Job Board
Training Courses Related to Data Integrity
GAMP® Data Integrity 21 CFR Part 11, 2-Day Training Course
The importance and amount of data being generated to ensure product quality and patient safety continues to grow, and proper controls around that data continue to be a subject of regulatory scrutiny. Regulatory agencies across the world are repeatedly citing data integrity issues. As a result, industry guidance and enforcement strategies are evolving. Regulatory concerns and warning letters have extended to all areas of the pharmaceutical, biotech, and medical device business, including manufacturing, development, clinical, pharmacovigilance and other areas of the product lifecycle. This…
GAMP® Data Integrity 21 CFR Part 11, 3-Day Training Course
This GAMP Data Integrity 21 CFR Part 11 Training Course will cover data integrity, electronic records and signatures, and the compliant operation of GxP Computerized Systems to provide the tools and techniques to implement proper controls for data.
Featured Conferences

Expert Xchanges
Upcoming
None Available
Past