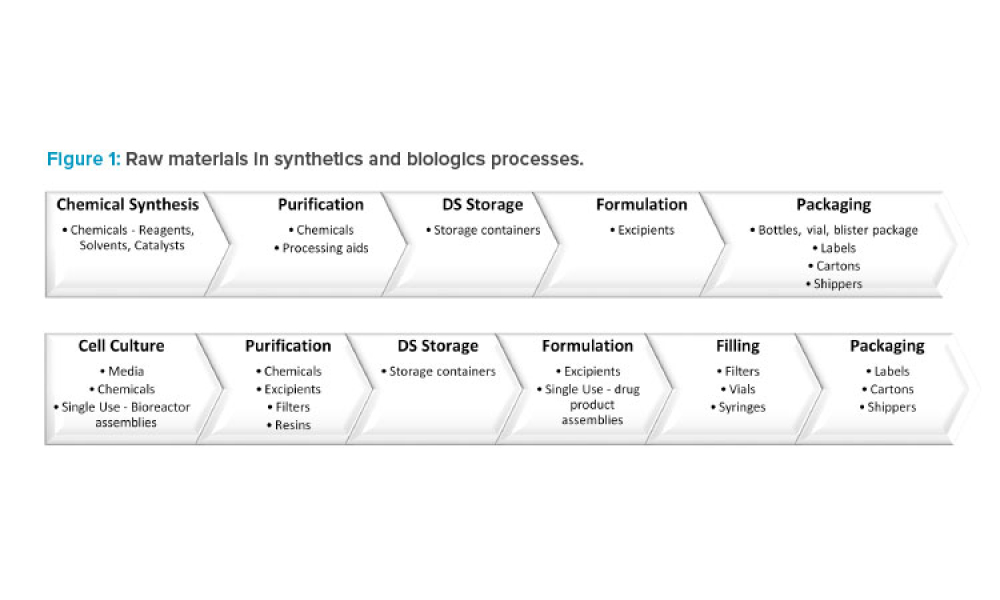
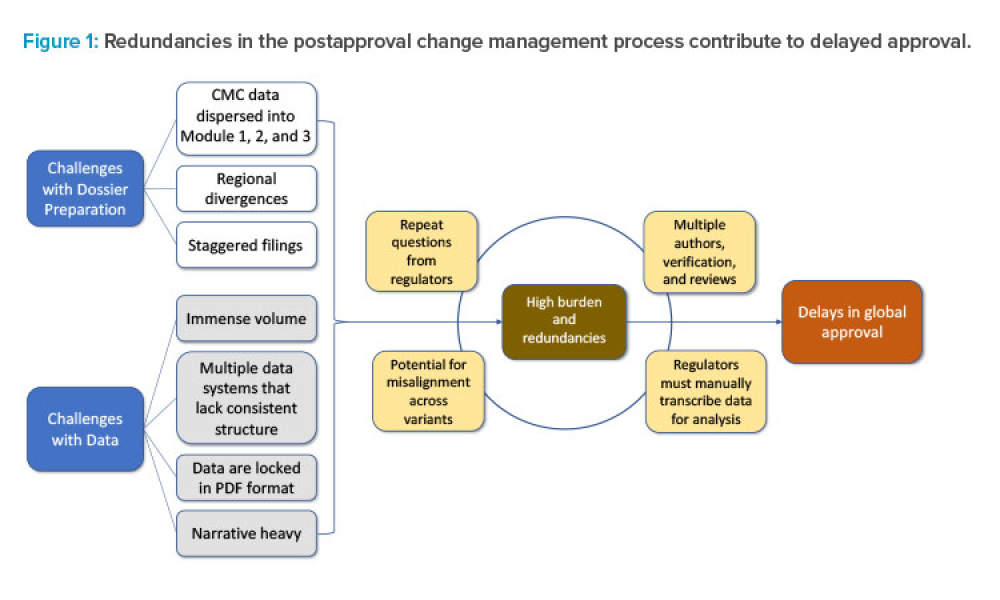
Leadership in regulation and quality affairs associated with ISPE core concerns and priorities
ISPE strives to facilitate industry wide clarity of new applicable regulations, advising on impacts and resolving towards solutions, seeking harmonization of regulatory expectations where desired and possible.
ISPE engages with all levels of regulators in the development and presentation of our education, training, document development, and interpretation to ensure that our offerings are cutting-edge and focused on clarifying issues and solving problems of importance to industry.
Learn MoreRelated Guidance Documents
Active Pharmaceutical Ingredients (2)
Advanced Manufacturing (1)
Advanced Therapy Medicinal Products (1)
Biotechnology (3)
Critical Utilities (6)
- Baseline Guide Vol 4: Water & Steam Systems 3rd Edition
- Good Practice Guide: Ozone Sanitization of Pharma Water Systems
- Good Practice Guide: Membrane-Based WFI Systems
- Good Practice Guide: Critical Utilities GMP Compliance
- D/A/CH Affiliate: WFI Handbook (English Translation)
- Good Practice Guide: Sampling Pharma Water, Steam, & Process Gases
Drug Shortages (2)
Good Manufacturing Practice (1)
Investigational Products (3)
Knowledge Management (2)
Lifecycle Management (2)
Manufacturing Operations (3)
Microbiological & Viral Contamination Control (7)
- Baseline Guide Vol 4: Water & Steam Systems 3rd Edition
- Good Practice Guide: Ozone Sanitization of Pharma Water Systems
- Good Practice Guide: Membrane-Based WFI Systems
- D/A/CH Affiliate: WFI Handbook (English Translation)
- Baseline Guide Vol 3: Sterile Product Manufacturing Facilities 3rd Edition
- Japan Affiliate: Pest Control Manual (English Translation)
- Good Practice Guide: Sampling Pharma Water, Steam, & Process Gases
Quality Assurance (2)
Quality Control (1)
Regulatory (5)
Sterile Products (1)
Supply Chain Management (2)
Community Discussions
Community Discussions
Nov 04, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Oct 30, 2024
Pharmaceutical Engineering Magazine Articles
Webinars Related to Regulatory
Concept and Discussion Papers
September / October 2023
Regulatory Trends & Quality Initiatives - Shortages of essential medicines around the world have…
January / February 2022
Emerging Leaders has grown from an initiative for interactions among early-career professionals and…
Unique Identification on Primary Containers to Drive Product Traceability & Quality
Health authorities and regulators are increasingly looking for much higher levels of traceability…
Remote Observation Technologies in the Pharmaceutical Manufacturing Space
1 Introduction Contemporary communications technologies, particularly those relating to the internet…
Use of Booklet Labels on Investigational Medicinal Products (IMPs)
1 Introduction It is current practice to use booklet labels for labelling of Investigational…

Videos Related to Regulatory
iSpeak Blog Posts Related to Regulatory
Featured Conferences

Expert Xchanges
Upcoming
None Available
Past
ISPE Expert Xchange: Distributed & Decentralised Manufacturing – Moving the Conversation Forward
Session Length:
1 hour 30 min

Training Programs Related To [Category]
CAPA / RCA / Investigations Training Course
Coming Soon 2024 - CAPA and Continuous Improvement using Process Performance & Product Quality Monitoring (PPPQMS), are elements of the Pharmaceutical Quality System (PQS), supported by ICH Q10. By practicing effective CAPA and PPPQMS a Pharmaceutical Quality System can realize Quality Management Maturity.
Pre-Approval Inspection Readiness Training Course
This new training course on the elements necessary to ensure readiness for pre-approval inspections provides strategy and tactics towards preparation than can be customized to individual organization requirements.
Implementation of ICH Q12 Guideline
Delivery Mode: Webinar ISPE Team Assists Training Health Canada with Implementation of ICH Q12 Guideline, Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management Regulatory Background International Council for Harmonisation’s (ICH) guideline entitled, “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management” (ICH Q12) provides a framework to facilitate the management of post-approval Chemistry, Manufacturing and Controls (CMC) changes in a more predictable and efficient manner across the product lifecycle. Implementation of ICH’s…
Biopharmaceuticals: CMC Aspects
This instructor led course is an advanced level course on Chemistry, Manufacturing and Controls (CMC) aspects of biopharmaceutical development. The purpose of this course is to provide an advanced understanding of the CMC considerations of biopharmaceuticals from development to commercialization. An emphasis will be placed on using Quality by Design principles to develop, manufacture and guide the product lifecycle. Lastly, we will examine biosimilars and regulatory factors that affect product development and the product lifecycle and drug costs.
Requirements for Computerized Systems Validation and Compliance
This online course describes regulatory requirements and expectations regarding the validation and compliance of computerized systems used in the manufacture of pharmaceuticals, biologicals, and medical devices. It does not cover the detailed requirements of 21 CFR Part 11, except for the requirement that systems be validated. Even though it draws upon medical device guidance, it is not intended to cover all the requirements of producing software that subsequently becomes part of a medical device.
Basic Principles of Computerized Systems Compliance
Basic Principles of Computerized Systems Compliance: Applying the GAMP5® Guide: A Risk-Based Approach to Compliant GxP Computerized Systems This fundamental online course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and explores tried, tested, and internationally recognized methods of meeting those requirements. GAMP guidance provides a pragmatic and effective framework for achieving computerized systems that are fit for intended use and meet current regulatory requirements, by building upon existing industry good practice in an…
View Course
Critical Utility GMP Compliance
This course is focused on how to be compliant in the design, operation & qualification of critical utilities and how to prove it. It comprises four modules including interactive case studies from real experience. This helps the participants to experience live discussions on compliance topics. The content is primarily based on the new ISPE Good Practice Guide “Critical Utilities GMP Compliance”.
Commissioning and Qualification Training Course
Worldwide Regulatory expectations and guidance as led by FDA and the EU have stated that all Pharmaceutical Quality Systems should apply a QRM (Quality Risk Management) approach. Through interactive workshops, this course will explain and apply the science and risk-based approach to integrated lifecycle Commissioning & Qualification by conducting verification of systems, equipment and facilities in accordance with the recently issued 2nd Edition Guide, ICH documents Q8 (R2), Q9, and Q10, current Regulatory Guidance, industry best practices, and ASTM E2500.
Process Validation Training Course
This training course is relevant to individuals working throughout the pharmaceutical product lifecycle in development, manufacturing, quality, and many other roles involved in validation of products and processes. It will help you integrate and link the science and risk-based lifecycle approach for Process Validation to your overall Pharmaceutical Quality System.
GMP Fundamentals for the Pharmaceutical Industry
This course is designed to help participants understand the GMPs as they relate to the pharmaceutical industry. Participants will gather information about both European regulators and the FDA, how the various agencies enforce GMP requirements, and what to expect during a routine GMP inspection. They will examine and discuss the process of a regulatory inspection and gain valuable insight into the compliance auditing process.