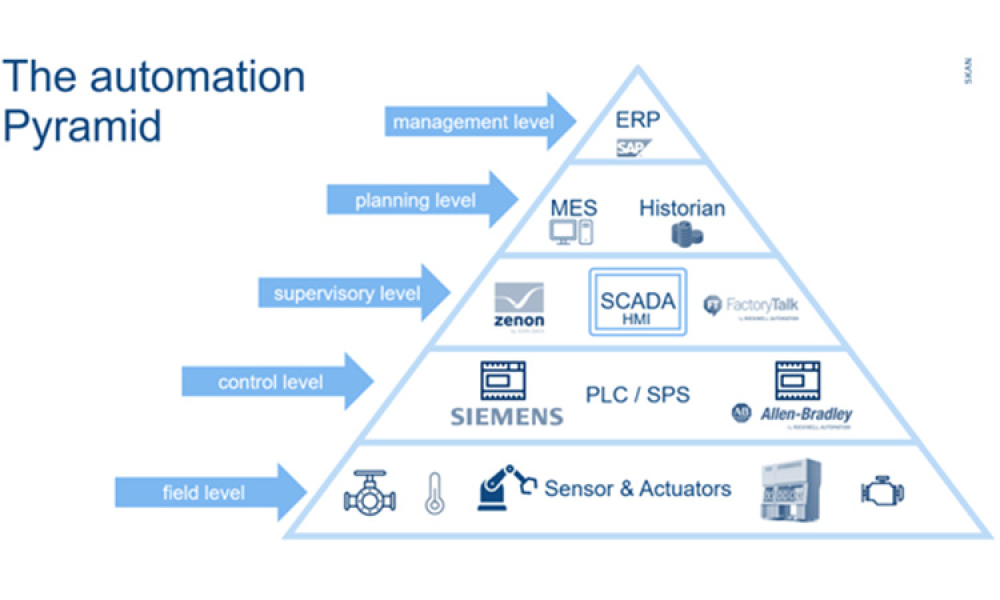
Quality Assurance
Quality Assurance (QA): Pharmaceutical quality assurance may be defined as the sum of all activities and responsibilities required to ensure that the medicine that reaches the patient is safe, effective, and acceptable to the patient.
Related Guidance Documents
Compounding (1)
Drug Shortages (1)
Good Manufacturing Practice (1)
Knowledge Management (4)
Project Management (1)
Quality Assurance (8)
- Guide: 503B Compounding
- Good Practice Guide: Process Validation
- APQ Guide: Cultural Excellence (Download) - USD
- APQ Guide: Change Mgmt System Download - USD
- APQ Guide: Mgmt Responsibilities & Review Download - USD
- APQ Guide: CAPA System Download - USD
- PQLI Guide: Part 3 - Change Management System
- Cultural Excellence Report Download - USD
Validation (1)
Community Discussions
Community Discussions
Nov 04, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Nov 02, 2024
Oct 30, 2024
Related Pharmaceutical Engineering Magazine Articles
Webinars Related to Quality Assurance
Concept and Discussion Papers
September / October 2023
Regulatory Trends & Quality Initiatives - Shortages of essential medicines around the world have…
July / August 2022
ISPE held an Expert Xchange on 18 January 2022 that included presentations and interactive exercises…

iSpeak Blog Posts
Training Programs Related To Quality Assurance
Advancing Pharmaceutical Quality (APQ) Quality Management Maturity Training Course
The ISPE Advancing Pharmaceutical Quality (APQ) Program has been developed by industry representatives, for industry use, to provide a practical framework that organizations can use to assess and advance the state of quality within their organization. The APQ program recognizes that the ability to advance the maturity of quality management lies within the industry itself and provides a range of sustainable and practical quality management improvement strategies.
GMP Fundamentals: Eleven-Part Bundle Series
Obtain a 10% Savings by Purchasing All Eleven Courses Overview ISPE is presenting an eleven-part series that will focus on the fundamentals of good manufacturing practices (GMPs). The series provides an overview of the regulations pertaining to GMPs and covers topics such as: manufacturing controls, product distribution, plant hygiene, documentation practices, buildings & facilities, organizational structure, and more. This offering gives users access to all eleven modules of the series and is intended to introduce GMPs for the new pharmaceutical employee or to provide an annual refresher for…
View Course
CAPA / RCA / Investigations Training Course
Coming Soon 2024 - CAPA and Continuous Improvement using Process Performance & Product Quality Monitoring (PPPQMS), are elements of the Pharmaceutical Quality System (PQS), supported by ICH Q10. By practicing effective CAPA and PPPQMS a Pharmaceutical Quality System can realize Quality Management Maturity.
GMP Fundamentals: Holding & Distribution
In this course, you will learn the requirements for storing, handling, and distributing finished products to ensure they are stored properly and not contaminated or altered during transport. For a limited time, registration fees are waived for this course. Interactive Course: What to Expect CEUs are provided once you achieve an 80% passing grade and complete the evaluation. Buy Now Return to Online Learning
GMP Fundamentals: Packaging & Labeling Control
In this course, you will learn how to ensure the safety and quality of pharmaceutical products using fundamental principles and best practices. During this course, you will learn about the FDA's pharmaceutical packaging and labeling guidelines. You will gain an understanding of the basics of packaging and labeling operations, including production and inspection processes, documentation, and record-keeping. Interactive Course: What to Expect CEUs are provided once you achieve an 80% passing grade and complete the evaluation. Buy Now Return to Online Learning
GMP Fundamentals: Production & Process Controls
This course offers an overview of Good Manufacturing Practices (GMP) for medical product production and process control. You will learn about GMP compliance, product design and development, process validation, control systems, and quality control procedures. Regulatory requirements, including FDA and international standards, will be covered. Upon completion, you will understand the fundamental principles and practices of GMP in medical product production. Interactive Course: What to Expect CEUs are provided once you achieve an 80% passing grade and complete the evaluation. Buy Now Return to…
GMP Fundamentals: Control of Excipients, Components and Drug Product Containers/Closures
In this course, you will learn about the fundamentals and importance of GMP control in the pharmaceutical industry. Good Manufacturing Practices (GMP), is critical for ensuring the quality, safety, and efficacy of pharmaceutical products. This includes controlling excipients, components, and drug product containers/closures to meet specific standards and requirements, reducing the risk of contamination, degradation, or incorrect formulation. You will gain a deeper understanding of the role of GMP control in maintaining the integrity of the supply chain, and how following GMP guidelines helps…
GMP Fundamentals: Equipment
GMP equipment is used in the production of pharmaceutical and medical device products. The proper design, function, qualification and control of such equipment is essential in the manufacture of pure, safe and effective pharmaceutical products. In this course learners will understand equipment design, size and location, cleaning & maintenance as well as the qualifications of GMP equipment. In this On Demand course (part 5 of 11), you will learn the elements of GMP equipment, as required by regulation. Interactive Course: What to Expect CEUs are provided once you achieve an 80% passing grade…
GMP Fundamentals: History
After a history of tragic events that affected public safety, the current Good Manufacturing Practices (GMPs) were developed. GMPs are a set of regulations that uphold the Federal Food, Drug, and Cosmetic Act. It is important to look back at the history of these events, their effect on public health and the FDA legislation that was set in place to protect consumers. This On Demand course (part 1 of 11) is designed to be a fundamental introduction to Good Manufacturing Practices. Participants will increase their understanding of the History of GMP regulations, the regulatory process, and the…
GMP Fundamentals: Organization and Personnel
The Code of Federal Regulations (CFR) states: "Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform the assigned functions. Training shall be in the particular operations that the employee performs and in current good manufacturing practice as they relate to the employee's functions. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuous basis and with sufficient frequency to ensure that employees…
Concept and Discussion Papers
Process Events for the Life Science Industry Information Model Concept on OPC UA for Alarms and Audit Trails
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
November / December 2023
There is much that large-scale commercial stem cell therapy processes can adopt from the existing…