Biopharma Adopts Mechanistic Modeling
Mechanistic modeling has been widely used in the petrochemical industry for many decades, while biochemical producers have been slower to adopt. The first biochemical simulators appeared in the mid-1980s.
More recently, regulators like the FDA have increasingly promoted the benefits of modeling (e.g., ICH Q8) throughout the drug development lifecycle.
These simulators enable key process design parameters to be rigorously assessed in terms of their impact on risk and performance. This in turn nurtures process understanding,
motivating companies to establish modeling as a key enabler for process design. Primary benefits include acceleration of the steps for design, scale-up, and regulatory approval for all process unit operations, in support of bringing new drugs to market in a timely manner.
Characteristics of a Modern Process Simulator
Modern biopharma process simulator offerings include a biocomponent databank that contains common cell lines used throughout the industry, coupled with a thermodynamic property method to evaluate properties such as enthalpy, density, or heat capacity of biological components. Another important component is unstructured macroscopic kinetic models that capture cell culture performance including biomass growth, substrate consumption, and both growth and non-growth associated product formation, enabling the rate of change of different biomass states to be tracked. In contrast to the structured approach, where the model explicitly considers cell physiology, an advantage of using the unstructured approach is that the biological reactions are lumped and defined as functions of variables such as nutrient and metabolite concentrations, simplifying the model in terms of the amount of data needed whilst still capturing the underlying cell culture phenomena.
A flexible simulator also allows users to append and customize the kinetic rate expressions as needed. For instance, custom terms can be used to account for the effect of different variables on the growth rate, such as pH, agitation speed, or even the presence of toxic metabolites that inhibit growth during cultivation. As an example, Figure 1 shows an input form for specifying fermentation kinetics.
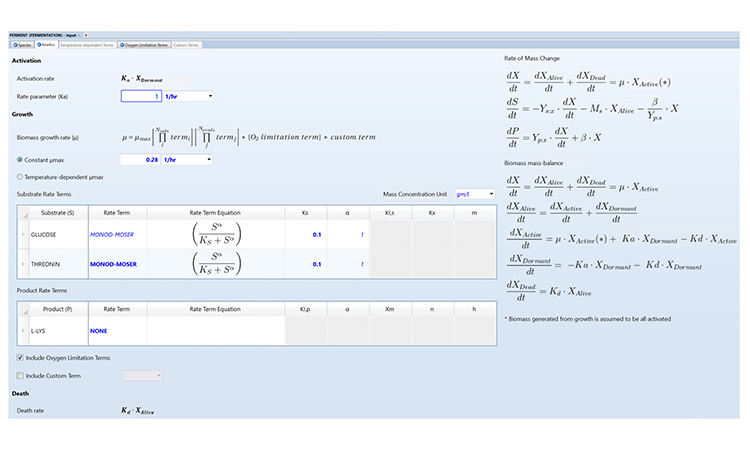
Integrated Design through Mechanistic Modeling
Traditional process design methods tend to sequentially tackle activities such as cell line selection, medium optimization, and the design of the bioreactor and purification unit operations, but this approach does not consider the interplay between these parameters, and opportunities for a fully optimized design are missed. By contrast, mechanistic modeling provides an integrated development workflow, grounded in physicochemical and kinetic models, that can optimize all of these design choices holistically. Underpinning this integrated approach, even if the whole fermentation process performance is not fully predicted, the embedded first principles enhance understanding of the governing relationships between the process and target variables.
Key fermentation results reported typically include viable cell density, product, and biomass titer, calculated specific growth rate for the cells, oxygen uptake, oxygen transfer rate from the gas to the liquid phase, CO2 produced during fermentation, and the overall mass balance based on elemental composition.
Also helpful is to have a holistic flowsheet environment for a quick yet rigorous tradeoff analysis, for example for assessing candidate cell lines, as well as comparing alternative bioreactor configurations such as full batch, fed-batch and perfusion modes, either for aerobic or anaerobic systems. This allows a comparative screening of design configurations and process conditions: the different configurations are defined through unit procedures, which consist of a sequence of batch execution steps; the settings of the variables associated with each material stream or fermentation reactor are optimized; the full batch schedule is visualized; and the evolution of the manipulated variables can be monitored via strip charts.
Mechanistic Modeling Supports Sustainability Initiatives
Process simulators for bioprocess modeling lend strong support to the Sustainability by Design framework, aimed to address environmental considerations throughout the product lifecycle.
This process simulation approach is especially advantageous during late-stage process development and manufacturing. For example, process simulators can report model-based air emissions such as volatile organic compounds (VOCs) with great accuracy since they are grounded in robust physical properties calculations, like activity coefficient models, that are embedded in the simulators. These calculations are then shared with the environmental agencies. Additionally, in support of industry initiatives to progress towards net zero manufacturing, process simulators can be used to assess greenhouse gas emissions in terms of CO2 equivalents of global warming potential (GWP), for both Scope 1 emissions, which represent the direct emissions of the process, and Scope 2 emissions, which relate to the comparative emission impacts of alternative utility choices.
Leading Biopharma Companies Are Embracing Mechanistic Modeling
Leading biopharmaceutical innovators and CDMOs are actively employing process simulation to enable efficient and more sustainable processes to be developed and approved with greater speed and for more optimized outcomes. For a more in-depth review of these topics, including Q&A, register for the ISPE webinar “Advance Biopharma Tech Transfer Using Process Modeling & Simulation”, on Thursday, 4 May 2023 at 1000 ET.
Register Now!
iSpeak Blog posts provide an opportunity for the dissemination of ideas and opinions on topics impacting the pharmaceutical industry. Ideas and opinions expressed in iSpeak Blog posts are those of the author(s) and publication thereof does not imply endorsement by ISPE.




