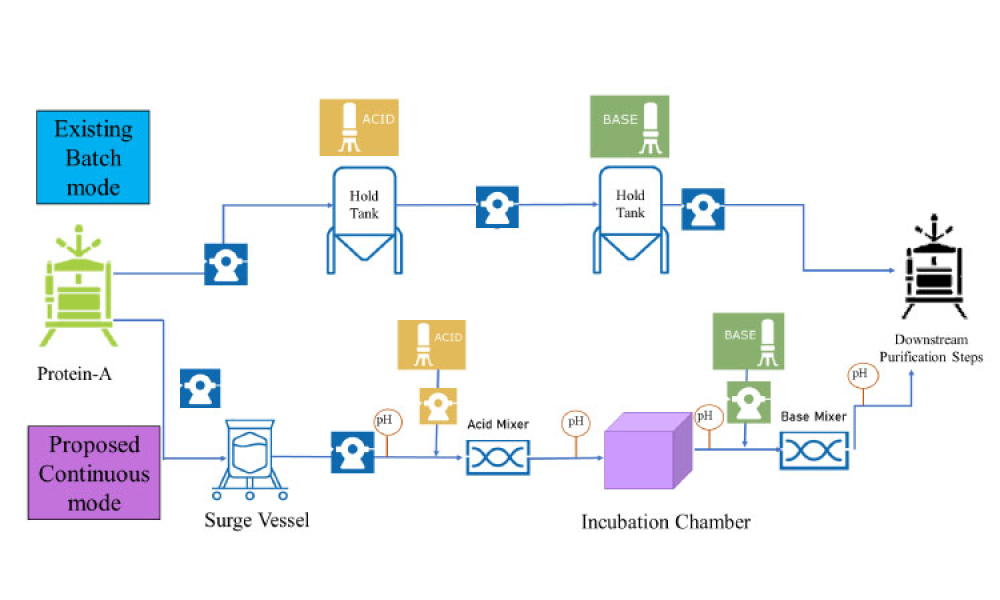
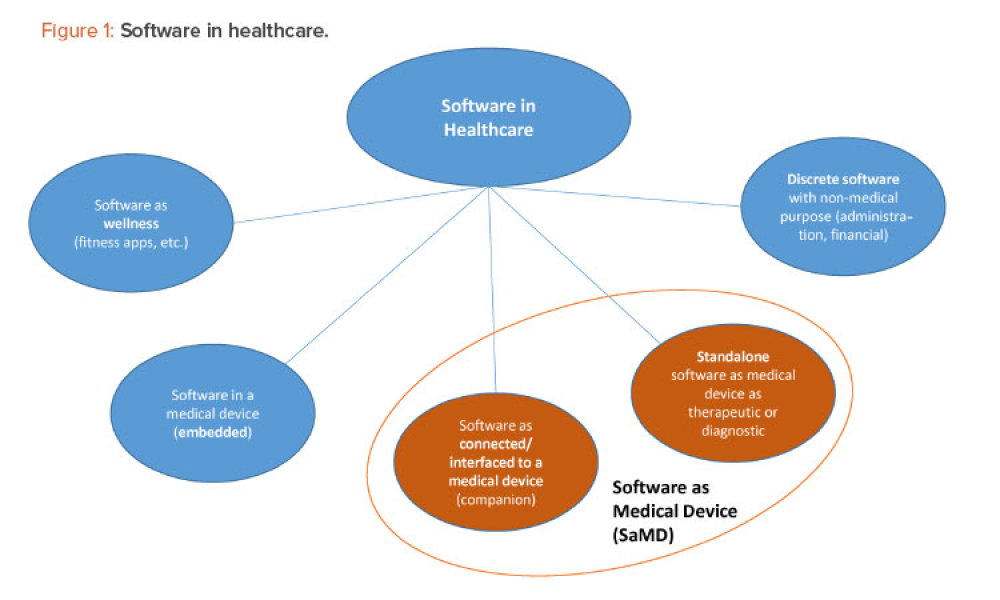
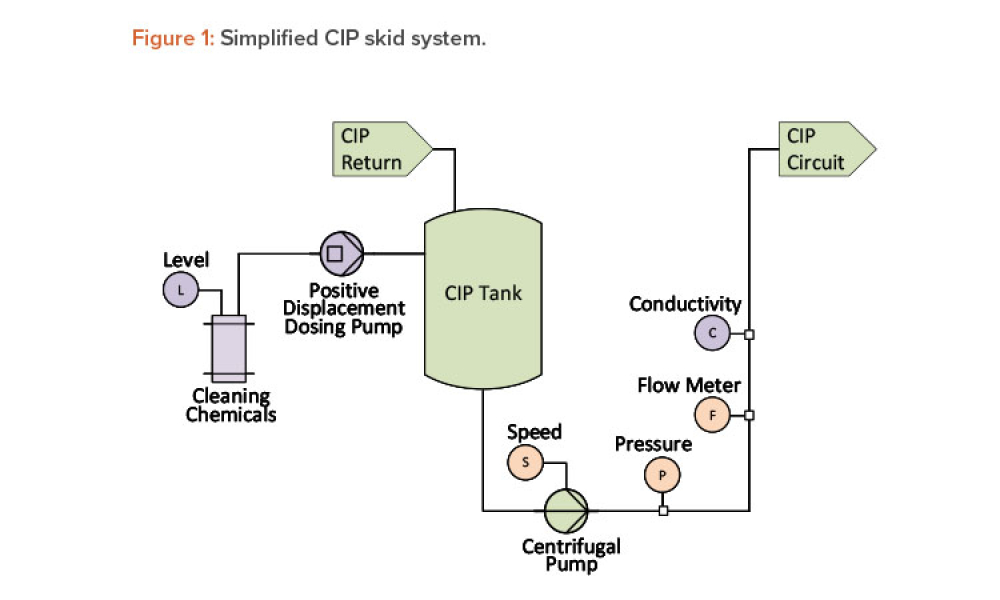
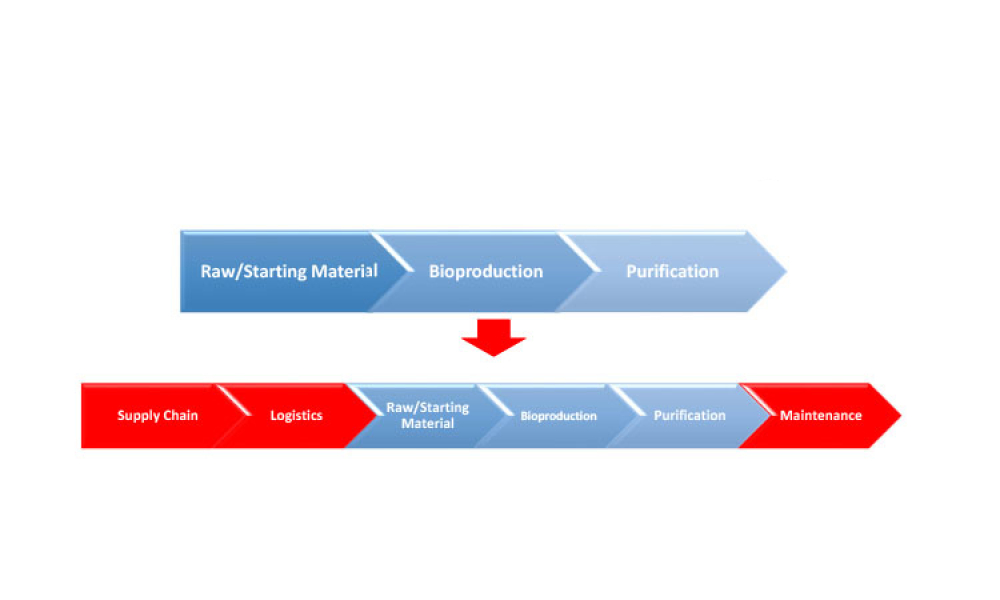
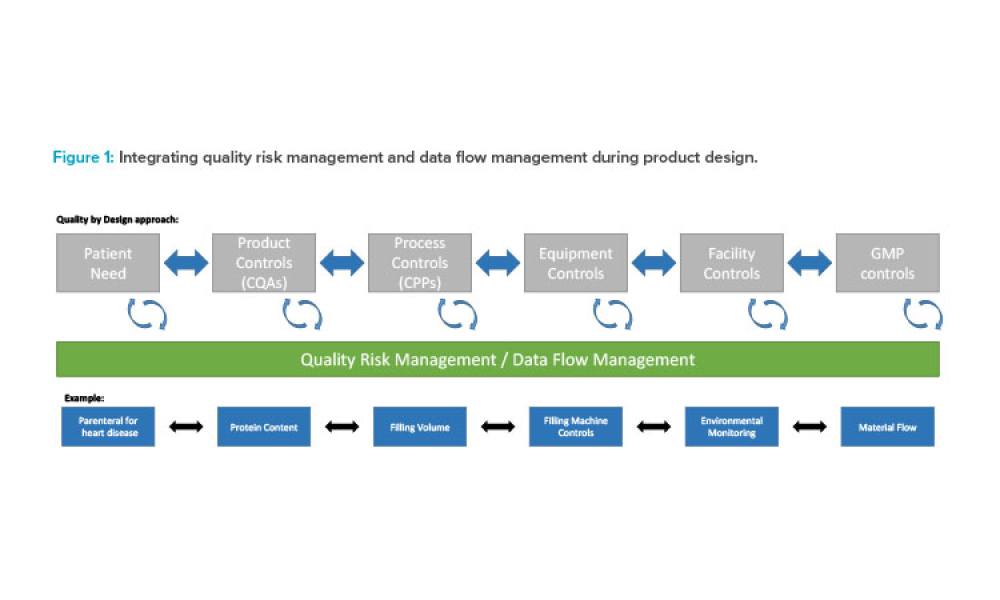
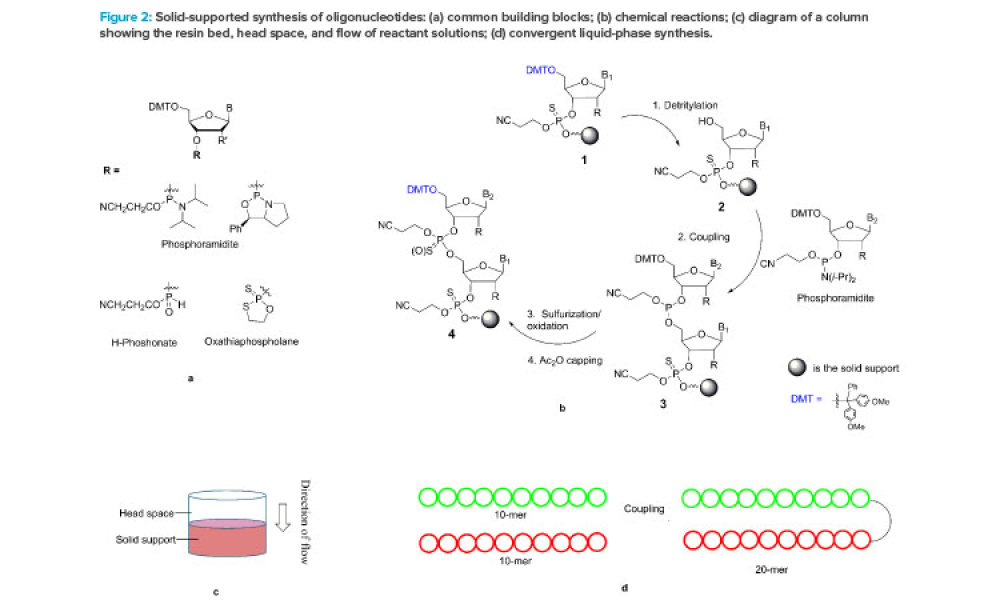
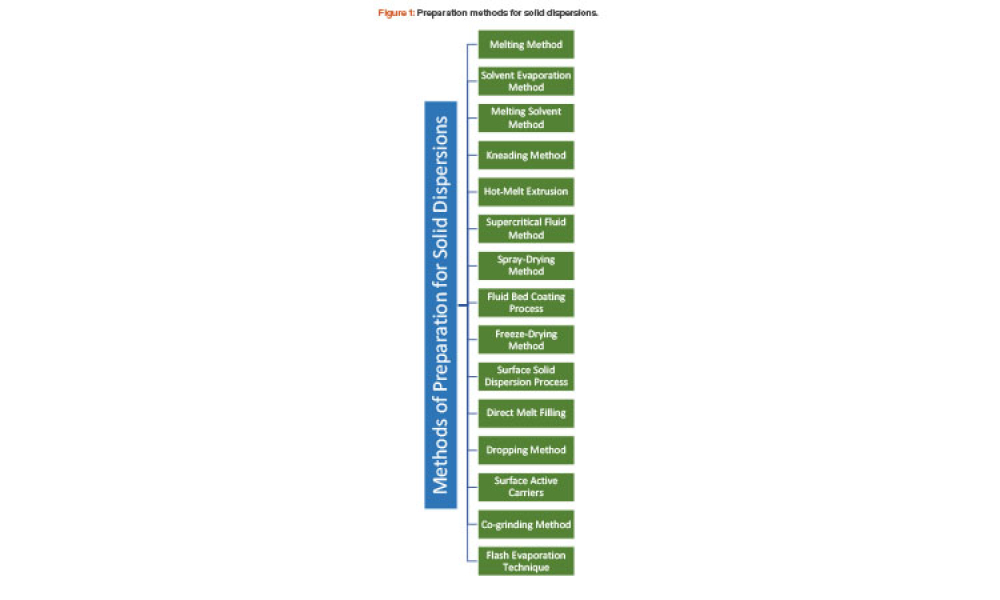
Live biotherapeutic products (LBPs) have the potential to treat a wide range of ailments. However, these living microorganisms are difficult to produce due to evolving government regulations and limited GMP manufacturing experience. New facility designs and more specific process guidance could help overcome these challenges. This article explores the nuances of facility design and regulatory...
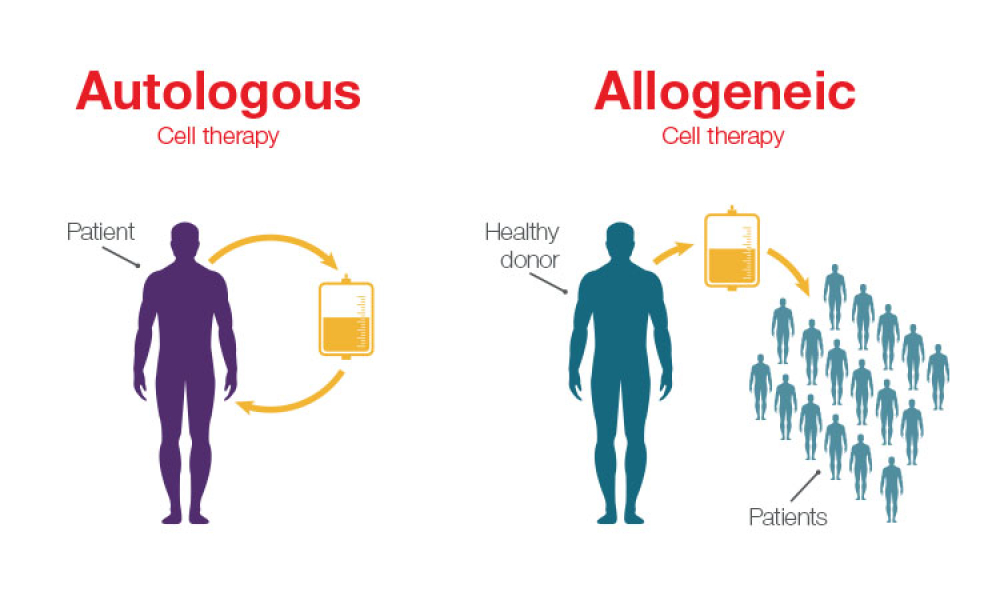
Cell and gene therapy (C>) products comprise a rapidly growing field of innovative medicines that hold the promise to treat and, in some cases, cure diseases that are otherwise untreatable. In this article, we provide points to consider when evaluating the comparability of C> when changes are made in their manufacturing processes.