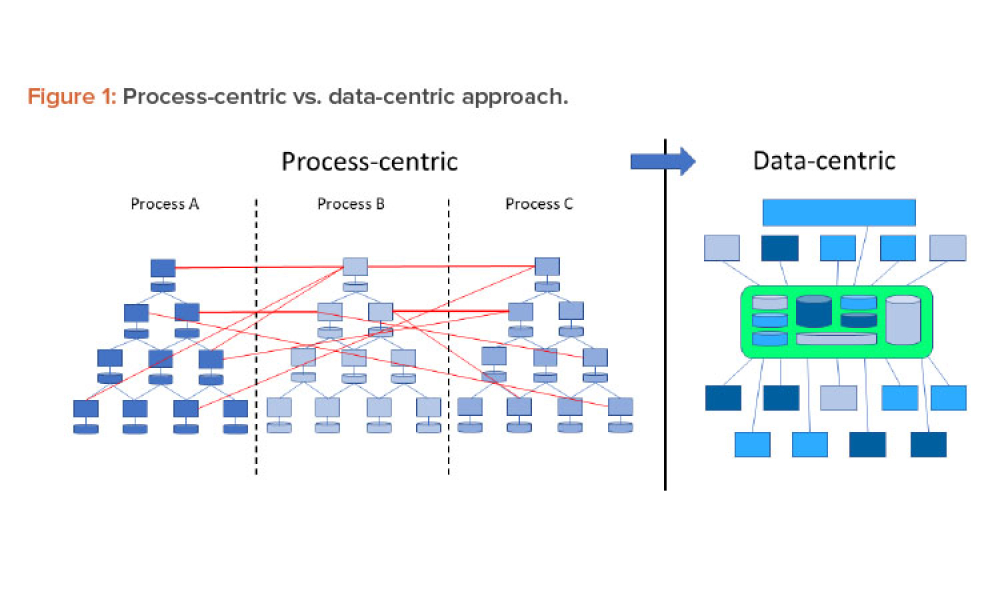
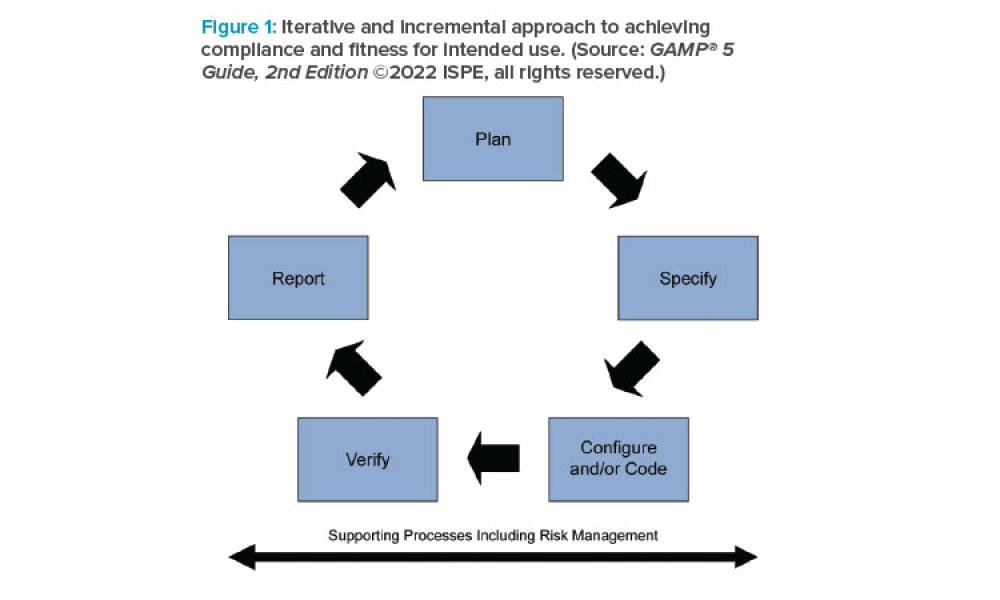
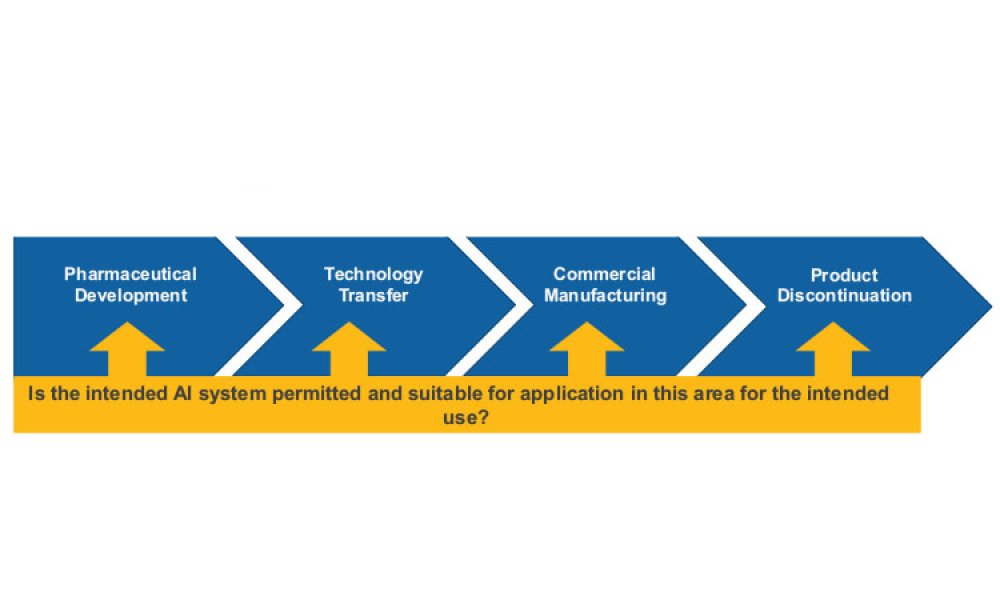
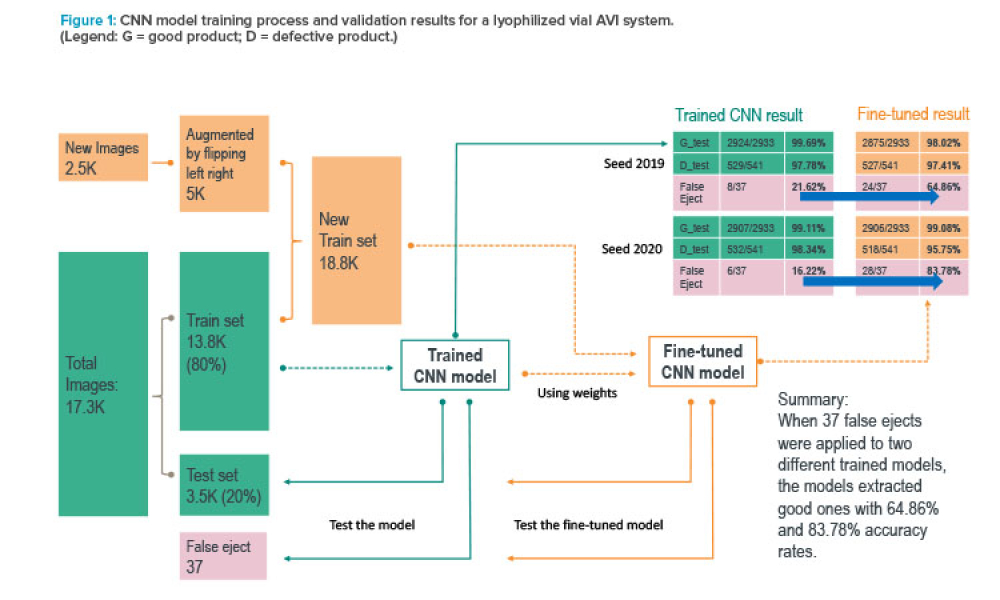
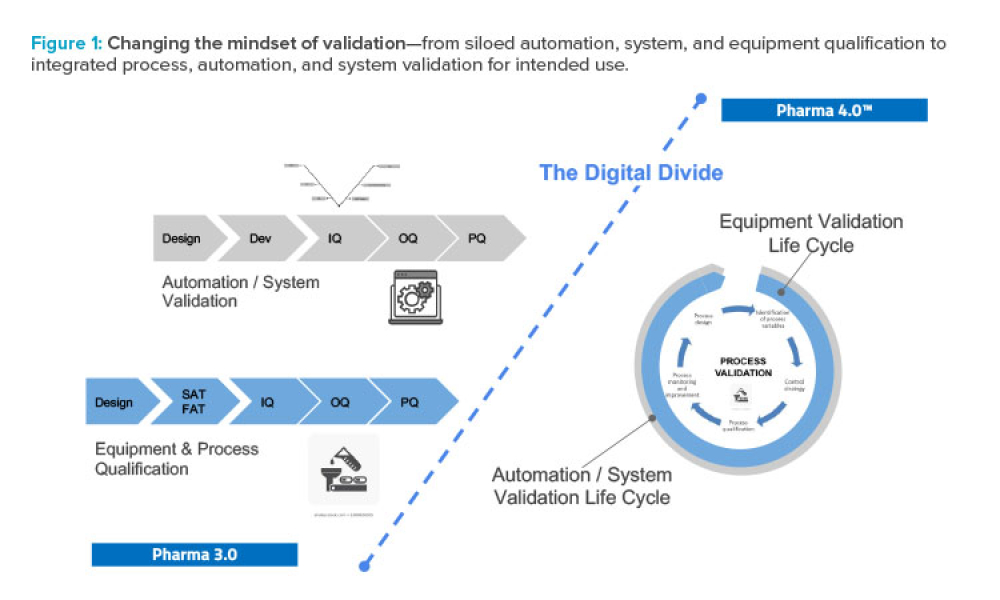
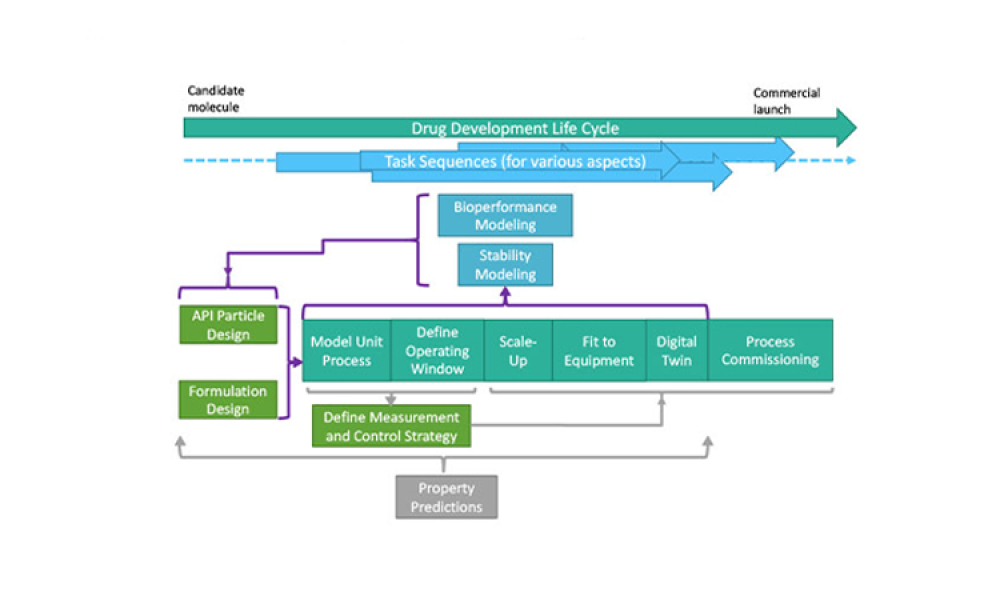
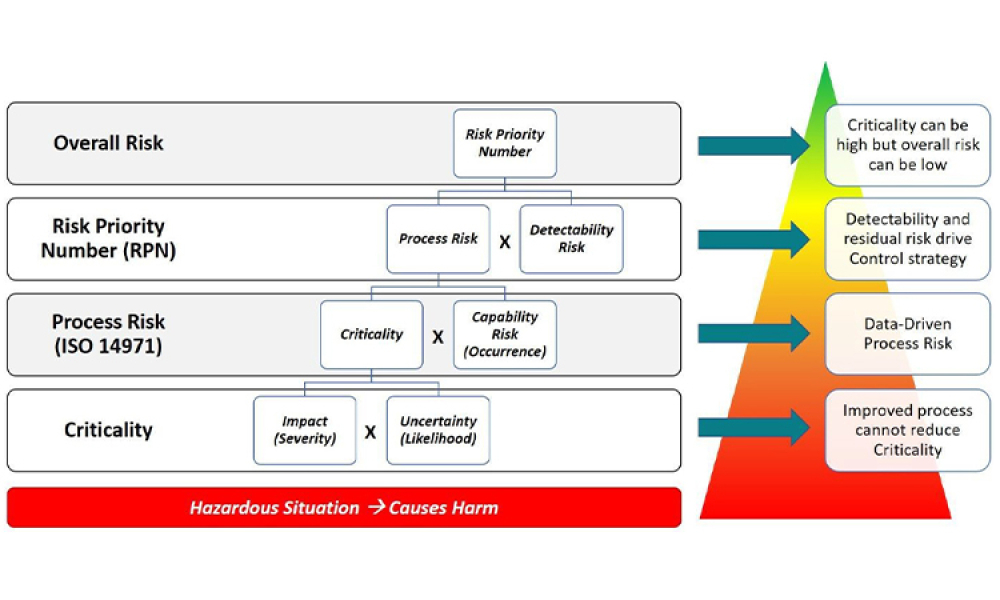
Stakeholders across industries are becoming accustomed to using information technology (IT) systems, applications, and business solutions that feature artificial intelligence (AI) and machine learning (ML). Even though some of these uses show phenomenal performance, thorough risk management is required to ensure quality and regulatory compliance are met within the life sciences industry. By...
Due to the growing digitalization of the industry, we are highly dependent on information technology (IT) systems and data. The basic ability to execute our pharmaceutical business and decision-making processes relies on the permanent availability of these IT systems and data to ensure compliance and efficiency of our business operations. But numerous factors—including criminal activities,...