Applying QRM to Improve Sustainability of Pharma Manufacturing

As economic pressures and legislated environmental protection measures continue to increase, the pharmaceutical industry is moving to control cost, reduce energy use, and become a better steward of the environment.
Within pharmaceutical manufacturing facilities, energy consumption due to the maintenance of environmental conditions through operation of heating, ventilation, and air-conditioning systems (HVAC) is typically the most significant energy user (often accounting for 50%–70% of the total energy consumption). Optimization and improvement initiatives in this area such as reducing the air change rates can provide some of the most significant opportunities for reduction in energy consumption, with the associated reductions in cost and carbon footprint. The importance and nature of the products we manufacture bring into sharp focus the need to ensure that manufacturing operations maintain robust, scientifically sound principles of good manufacturing practice (GMP).
As energy costs and demands for carbon footprint reduction increase, it is critical we demonstrate the ability to appropriately control our manufacturing operations. Our patients and regulators must be assured that, regardless of these outside demands, our intent to avoid unacceptable risks to patient safety, product quality, and regulatory compliance remain steadfast.
The intent of this article is to provide discussion, guidance, and examples on the use of ICH Q9: “Quality Risk Management (QRM)” when reducing HVAC air change rates within manufacturing and supporting operations.
HISTORY
Typically during the design of new pharmaceutical manufacturing facilities and their associated HVAC systems, it has been simpler, cheaper, and more effective from a project timeline basis to focus on the process engineering and the development of the design space around the process. Environmental control is viewed as a secondary concern, and it is common practice to take a standardized, well-understood cleanroom solution (such as a formally classified area) and apply this to the scope of the project. While from both an engineering and quality perspective this may have been a wholly appropriate decision at the time, the chosen solution may not necessarily have been the most economic option.
This approach has resulted in a large number of areas having significantly higher air change rates than is required to maintain an environment complying with the regulations and product /process requirements.
The problem, we suggest, is the lack of a comprehensive understanding of the regulatory requirements and the science behind the provision of an effective design to provide appropriate effective environmental control via the HVAC system.
It is not unusual to see a minimum air change rate as one of the design criteria. Guidance values for air change rate are frequently misinterpreted as requirements. For example, the US Food and Drug Administration (FDA) guidance for sterile drug products suggests at least 20 air changes per hour (ACH) are typically acceptable to maintain ISO 8 (class 100,000) conditions during operations. This figure of 20 ACH is often quoted as a minimum ventilation rate for all cGMP facilities. Scientifically, depending on the particle challenge from the specific process and the efficacy of the HVAC design, the actual air change rate required may be as low as 6, 10, or as high as 30 ACH.
Qualitative assessments in evaluating cross-contamination risk often overstate the potential for airborne contamination, resulting in unnecessary 100% fresh air systems, HEPA filters, airlocks, and room-pressurization systems.
Today’s manufacturing projects face new challenges for designing, implementing, and managing facilities. The desire is to provide effective and efficient cross-contamination prevention with the appropriate understanding, control and management of risk.
It is clear that an effective, proactive process of risk management is required to balance product quality with the constraints of cost, environmental pressure, and regulatory compliance.
USE OF QRM IN HVAC AIR CHANGE REDUCTION
To demonstrate control of risk in any situation, one must first understand the existing risks. Effective risk management, including risk mitigation, can then be determined and applied. ICH Q9 “Quality Risk Management (QRM)” provides us with “a systematic process for the assessment, control, communication, and review of risks to the quality of the drug (medicinal) product across the product lifecycle.” 1
QRM is the appropriate process to use when developing the control processes to be used for cross-contamination prevention in facilities and manufacturing. It allows the definition of the risk, development and assessment of the efficacy of the controls to be effectively demonstrated to a regulator.
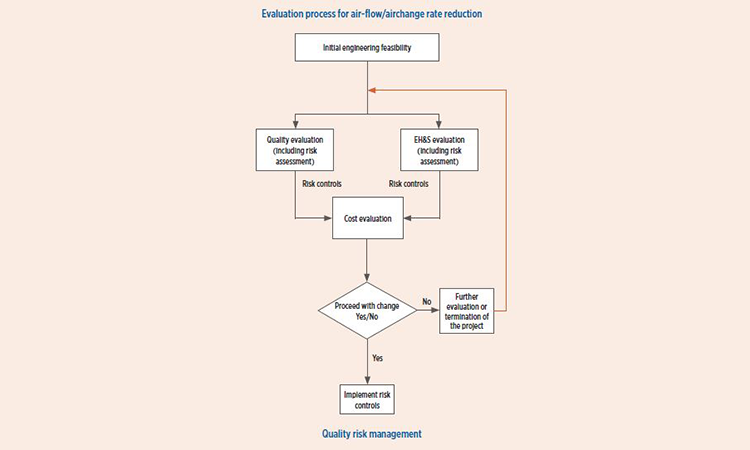
Application of QRM process
Figure 1 is intended to provide a simple overview of the use of QRM in HVAC air-change rate reduction. As can be seen, three key factors for consideration of HVAC reduction are:
- Product quality
- Environmental health and safety (EH&S)
- Costs associated with making the proposed change
The title “Quality Risk Management” may imply only quality considerations would be evaluated, but QRM provides the framework to apply the principals of risk management to each key factor.
Product quality evaluation
If initial engineering identifies opportunities for reduction in areas where GMP products or materials are manufactured or stored, the impact of the proposed changes to quality, patient safety, and regulatory compliance must be assessed.
- Microbiological risk assessment and evaluation will be incorporated into the QRM exercise. If microbiological attributes are considered, involvement of relevant technical or subject matter experts are required.
- If the HVAC system is operating under GMP change control, modifications made to the HVAC system must be made in accordance with relevant change control procedures.
- If changes are identified during initial new facility design or upgrade proposals for existing facilities, the risk assessment of the design can be conducted during any stage of the process, though the optimum approach will be to do it early, revising it as necessary during the design development process. Technical and quality representatives should be consulted regarding the new design proposals. Quality approval is incorporated as part of the new or upgrade design phase (design qualification).
The QRM process, including review and communication, is designed to identify the risk control measures that, when implemented, adequately reduce risk to an acceptable level, so that the proposal can proceed. If the risks cannot be reduced to an acceptable level, the proposal cannot proceed.
EH&S evaluation
When opportunities for reduction are identified, EH&S risks should be identified, evaluated, and mitigated when necessary. It is recommended EH&S evaluations be incorporated, either directly or by reference, in the overall QRM conducted for proposed changes. The site- or project-specific person responsible for EH&S should be involved in the risk assessment and use applicable tools, such as HAZOP (hazard and operability analysis), as required. Examples of EH&S risks include the potential for higher than OEL levels of solvent (due to process requirements, or cleaning/sanitization regimes) resulting from reduced air change rates—and the associated reduction in fresh air supply.
The EH&S risk management process should identify risk control measures to sufficiently reduce risk and allow changes to proceed. If the risks cannot be reduced to an acceptable level, the proposal cannot proceed.
Cost evaluation
The third factor to consider as part of the airflow/air change rate reduction initiative is potential or actual costs associated with implementing the proposed change. Apart from initial cost saving estimates determined within the pre-evaluation phase, other costs must be considered in the overall evaluation of the airflow/rate change reduction initiative.
Associated costs for consideration include, but are not limited to:
- Reduction in energy costs
- Cost of implementation, including required equipment modification cost
- Ongoing maintenance costs
- Operational costs (e.g., additional facility cleaning, increased start-up time)
- Analytical support cost (e.g., requalification, changes to routine environmental monitoring)
- Cost of failure (e.g., system fails to maintain minimum requirements and product quality, patient safety, or delayed regulatory compliance)
EVALUATION OF HVAC REDUCTION
Requirements to modify each HVAC system should be accurately defined and fully understood. The most commonly used scientific basis for developing an appropriate HVAC system design is specifying air quality requirements for the products manufactured in the areas defined.
Typical considerations for air quality requirements include:
- Viable and nonviable particulates: expressed as total colony forming units per unit assessed or total particulate count per unit volume of air
- Relative humidity: as applicable per specific product or EH&S requirement
- Temperature: as applicable per specific product or EH&S requirement
- Pressure cascade: as applicable per GMP and / or EH&S requirement
Typically a conservative set of HVAC parameters are selected to achieve the air quality requirements during design. These airflows and set point parameters are then used as a basis for demonstration and qualification to verify the HVAC system meets the design requirements.
A perceived positive result of this overly conservative design approach is that the resulting manufacturing areas often operate several orders of magnitude “cleaner” than specified (or required). The initial (through higher capacity equipment, larger ductwork, etc.) and ongoing operational cost required to achieve and maintain these very high levels of performance are wasted, as the over specification is typically such that the resulting additional control is not needed to comfortably achieve the specified product quality attributes and associated regulatory compliance (Figure 2).
For new facilities the designer should be tasked with defining the appropriate air change rate; for existing manufacturing areas current operational conditions and airflow rates should be considered and a risk assessment conducted where the operational information shows that there are opportunities to optimize the operation. Examples of drivers for this change may include:
- Reduce energy consumption and associated costs
- Reduce noise
- Decrease lifecycle cost of the HVAC system
- Decrease site utility loads to avoid upgrade of utility generation capacity
- Improve understanding of quality and operational risks by reevaluation of the system
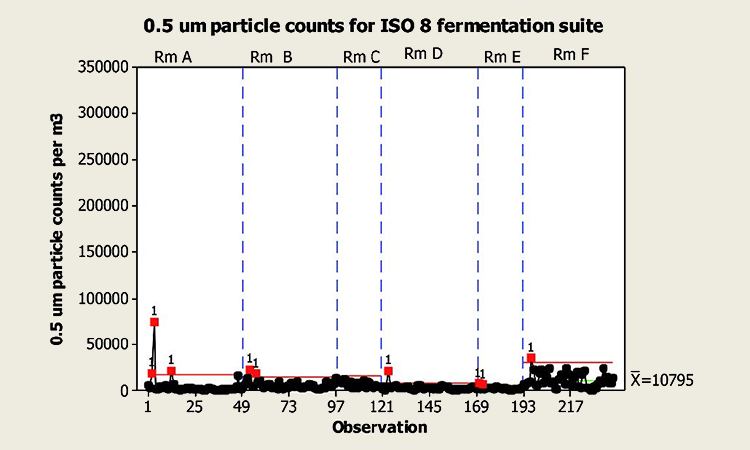
A sample of 0.5μm particle data from a fermentation are area classified as ISO 9 (in operation, ISO 8 at rest) is shown to left. Particle counts are may order of magnitude within the requirement for at rest, while the area is in fact in operation (class limit: 3,520,000 0.5 μm per cubic meter) ISO 8 fermentation suite – footer content of diagram
Prior to conducting a QRM exercise, it is recommended that the proposed change is pre-evaluated by the engineering department, with the potential benefits of the proposed change(s) quantified. This initial engineering feasibility study is conducted to determine if it is worth the investment required to conduct the QRM exercise.
A reduction in airflows or air change rates only can be considered if an appropriate risk assessment is conducted and approved. The approval should include site engineering, production, EH&S, and quality. The risk assessment should consider relevant and available data for the area and associated HVAC system, including operation and maintenance of room/area environmental parameters identified as critical to product quality and maintenance/production operator safety.
Where operating/maintenance data are not available, for example, in the design phase of new rooms/areas, this should be taken into account during the risk assessment, as unknown risks default to high. The outcome of the initial risk assessment may include actions to generate data, for example, proposed system-modeling (CFD) and/or baseline particulate monitoring. These data can then be used to reevaluate the risks associated with the proposed change or new design.
Special considerations
Rooms with special requirements, such as very low humidity or the need to recover quickly after room cleaning so production can resume, need additional evaluation to determine if air-change reduction is feasible.
Engineering evaluation process
As mentioned earlier, the first step in the evaluation of air-change reduction is to perform an engineering feasibility study and high-level estimate of possible operating cost savings and enabling costs. After the risk assessment step, the cost estimate should be updated to include the cost of mitigating measures and the cost vs. benefit reviewed again. Except for very simple, straightforward situations, there is often a “detailed design” phase when the proposed reduced air change rates are compared against the actual individual room requirements for air to maintain room conditions and pressurization, etc., system fan and control device turndown is analyzed, any equipment changes are determined, final new flow rates are calculated, and drawings are updated. Occasionally, tools such as airflow modeling are used for rooms where there are concerns about the ability to provide effective ventilation of critical parts of the room at reduced airflows. An updated estimate of implementation costs and projected operating cost savings is often done at this stage before a final decision is made to implement the changes.
Although each system and requirements are different, the evaluation stage and the later implementation stage can be greatly assisted through the development of a standardized process for evaluation, execution, and verification.
CONCLUSION
Reduction in air change rates for HVAC systems serving pharmaceutical facilities provides one of the most significant potential opportunities for energy reduction, with associated reduction of operating cost and carbon footprint. It also can provide benefit in terms of reduced equipment maintenance. When applied at the design stage for new facilities, additional benefits can be gained through lower initial capital cost of smaller equipment, including central utilities equipment such as boilers and chillers and their associated distribution systems.
When analyzing proposed changes there are many aspects that must be considered, and robust processes are needed to ensure that these aspects are effectively assessed, and that the specific and general requirements of the end user are met. This is, after all, about ensuring that the required environment is maintained to support the manufacturing process.
Use of the QRM approach provides an effective method to ensure the requirements from all stakeholders in the process are identified and assessed. Applying the QRM process results in a good understanding of what is really required of the HVAC system supporting manufacturing operations. The knowledge gained by using the QRM process forms a sound basis for optimizing the HVAC (e.g., air change rates) while properly controlling risks to patients, product quality, and maintaining regulatory compliance.