How to Do Bioprocess Characterization Studies Efficiently

Process characterization is an essential step in the commercialization of a new (biological-) drug. For drug product commercialization, manufacturers must validate the drug’s manufacturing process. This ensures, that the manufacturing process delivers consistently a quality product and that the patient is not at risk.
Process Validation is defined as the collection and evaluation of data, from the process design stage throughout production, which establishes scientific evidence that a process is capable of consistently delivering quality product FDA process validation guideline
Recently, US and European regulators have issued process validation guidelines which emphasize:
- the demonstration of process understanding;
- risk-based identification of critical process parameters;
- implementation of well-validated control strategies.
As a result of the new guidelines, it is now state of the art that drug manufacturers thoroughly investigate and “characterize” the manufacturing processes. Interestingly, the term “process characterization” is not used by the regulators. You will not find it in the EMAs and FDAs process validation guidelines. Yet, CMOs and (bio-) pharmaceutical companies use the term “process characterization” to describe their activities related to stage 1 process validation.
Why do manufacturers run process characterization studies?
Reason 1: Achieve compliance. Ultimately, the product should reach the patient. To achieve this, manufacturers must validate the manufacturing process. Process characterization is an integral part of stage 1 process validation.
Reason 2: Avoid registration delays. A delay in the commercialization of a product has negative impact on patients: They have no access to a drug they can benefit from. Furthermore, a delay in the commercialization costs money.
Reason 3: Avoid failed batches. Failed commercial batches in biologics manufacturing cost companies millions of dollars. A thoroughly characterized process with the right control strategy in place avoids deviations and failed batches in commercial manufacturing.
Goals of process characterization studies?
- identify process parameter that impact onto product quality and yield;
- justify manufacturing operating ranges and acceptance criteria;
- identify interactions between process parameters and critical quality attributes;
- ensure that the process delivers a product with reproducible yields and purity.
Do I need to include data from commercial scale in my process characterization study?
Yes, because you need to qualify your scale down model. Scale-down model qualification is an integral part of process characterization studies. To perform a scale down model qualification, commercial scale data (at set point conditions) is necessary.
What do I need to do? Statistical data analysis for process characterization studies
Biotech companies realize that data science is crucial to address following questions for successful process characterization:
- How to I use available data to support risk assessments (e.g. how to support failure mode and effect analysis)? - You may use deviation probabilities underpinned to each CPP.
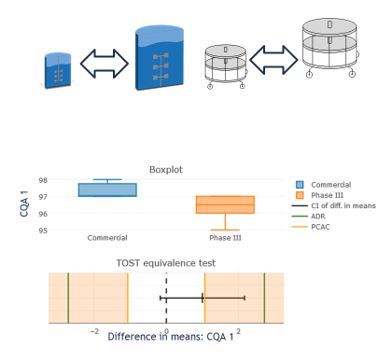
- How do I statistically demonstrate that my scale down model is appropriate? – TwoOneSidedT-Tests (TOST-tests)
- Which response variables should be studied in an experimental design in which unit operations? – Produce a Heat Map which CQAs are critical in which Unit Operations
- How do I state statistically that a parameter is key, non-key, critical or non-critical? How do I statistically define normal operating and proven acceptable ranges? – Use Power Analysis - http://www.mdpi.com/2306-5354/4/4/85
- How do I stack together multiple DoEs to identify optimization potential and predict out of specification (OOS) events? Use integrated process models / digital twins! http://www.mdpi.com/2306-5354/4/4/86
What are the prerequisites to do so? Data organization in process characterization studies
Authorities stress the importance of data collection in the process validation guidelines (FDA process validation guideline). Essential activity in process characterization is the collection of (non-GMP) data to support the regulatory filing.
This includes data from
- upstream (fermentation)
- primary recovery
- downstream
- quality
- small scale (laboratory)
- manufacturing scale
- commercial-like runs
- DoE experiments, one factor at a time experiments along ICH Q8 – Design Space
- … and many more
Hence as prerequisite for process characterization studies we need the following:
- Strategies to contextualized data structures and types, which are highly complex (time series data, one-point quality data, SDS page scans…).
- Links to data from laboratory (small scale experiments) and large scale manufacturing needs to be established for joint analysis.
- Data originating from a broad selection of legacy systems need to be aligned (laboratory equipment, LIMS systems, large scale manufacturing process control systems, manufacturing execution systems).
As conclusion, suppliers from the automation pyramid, data management, and data analytics need to collaborate on holistic data management and data analytic tools to avoid interfaces via Excel and endanger data integrity. This will allow manufacturing organizations to speed up regulatory filing and accelerated commercialization!
Want to get more information like what you read above? Or have specific questions that you’d like to get answered? Join ISPE and take advantage of the Communities of Practice, which gives you access to pharma professionals from around the world who have similar job functions that you can collaborate with on topic-specific discussions.



