How to Minimize Impact on Overall Equipment Effectiveness (OEE)

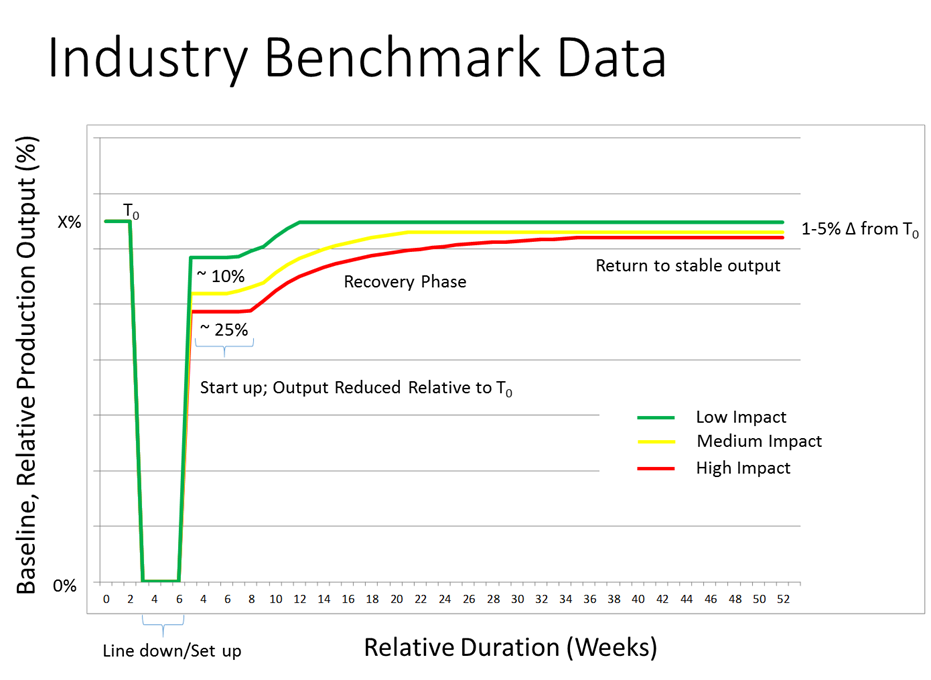
Overall Equipment Effectiveness (OEE) is a key metric for Packaging Operations. When used well, it can help determine the effect of continuous improvement and when it is the right time to add new capacity or replace existing equipment. It is not surprising that overall equipment effectiveness (OEE) is often raised when deploying Serialization. It is important to understand the impact on OEE, and more importantly, how to prevent any impact on the supply of product. It is widely assumed that Serialization negatively impacts OEE which is supported by industry benchmark data. However, does Serialization always reduce OEE and if it does how can it recover over time? This post explores OEE impact due to serialization, how to recover from that impact and maintain the supply of product.
In most cases serialization will result in a drop in overall equipment effectiveness (OEE). However, there are cases when the OEE does actually increase. For example, when replacing existing equipment, the OEE can increase due to more modern technology and higher equipment speeds resulting in less downtime or minimizing product quality issues. For example, when replacing Case Packers on a line that are not suitable for Serialization. Selecting the right solution is critical to restoring the OEE. Establishing core solutions is a pre-requisite and will help ensure industry best practices are upheld when deploying lines. Deviating from industry best practices may result in a significant loss in OEE. For example, attempting to print a China 1D barcode on-line using a thermal inkjet printer will impact OEE considerably more with less opportunity to recover, than perhaps using laser printing technology. It is worth noting that higher volume lines with fewer SKU’s can often result in a much quicker recovery to previous OEE levels.
An effective way to minimize the reduction in overall equipment effectiveness (OEE) is to recognize there will be an impact and to plan accordingly for that impact, e.g. by extending the shutdown period when upgrading a line and increasing the amount of testing performed. For critical high-volume lines, it may be necessary to perform multiple shutdowns to avoid stock-outs and to maintain supply to a market. For example, begin with a shutdown to enable unit serialization followed by a second shutdown to enable aggregation three or four months later. If enabling serialization at a site for the first time, then it is highly recommended to enable serialization at least 6 months prior to the enforcement date. The learning curve is always underestimated, and problems encountered at each site are often slightly different and difficult to plan for.
The biggest risk to overall equipment effectiveness (OEE) is when a serialization solution is retrofitted to existing equipment (which is a common approach due to the high capital cost of replacing packaging equipment). When doing so, it is important the Original Equipment Manufacturer (OEM) & Serialization Solution Provider develop the integration guides together. It is important to perform detailed and comprehensive testing on site and to not underestimate the amount of training required. If running serialized and non-serialized SKU’s on a line, make sure the packaging line can operate with non-serialized SKU’s as before. It is not uncommon for non-serialized product to be impacted more by serialization if supplying product to multiple markets and especially if the volumes for non-serialized markets are much higher. Although costlier, pre-printing the cartons or labels with the 1D or 2D barcode can result in a more reliable & robust packaging operation.
In summary, there are many factors that impact overall equipment effectiveness (OEE) and many steps that can be taken to minimize the impact and ensure the supply chain is not affected.
Want to get more information like what you read above? Or have specific questions that you’d like to get answered? Join ISPE and take advantage of the Communities of Practice, which gives you access to pharma professionals from around the world who have similar job functions that you can collaborate with on topic-specific discussions.


