On 20 November 2019, the ICH Assembly endorsed the Q12 guideline, “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management,” at its biannual meeting in Singapore. This transformational guideline has a wide scope of applicability across pharmaceutical drug substances and products (both chemical and biological), drug-device combination products that meet the...

Downloads
Regulatory Trends: ICH Q12’s Impact on Product Life-Cycle Management
Introducing ICH Q12: A Transformational Product Life-Cycle Management Guideline
Cover: The ICH Assembly endorsed the Q12 guideline, “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management,” in November 2019. This transformational guideline has a wide scope of applicability across pharmaceutical drug substances and products (both chemical and biological), drug-device combination products that meet the definition of a pharmaceutical or biological product, as well as both new molecular entities and authorized products.
ISPE Comments to the Annex 2 PIC/S Draft Revision
Feature: Annex 2 is the GMP document by the Pharmaceutical Inspection Co-operation Scheme (PIC/S) addressing manufacture of biological medicinal substances and products for human use. This article shares information about Annex 2 and ISPE’s submitted comments to the draft revision of Annex 2 that was released for public consultation in September 2019.
Update from the ISPE Regulatory Steering Council
Feature: The ISPE Regulatory Steering Council is an advisory group whose primary responsibilities are to develop, prioritize, and reconcile regulatory policy issues through ISPE on behalf of the pharmaceutical industry. This article shares the RSC’s latest initiatives and current goals.
Risk Management
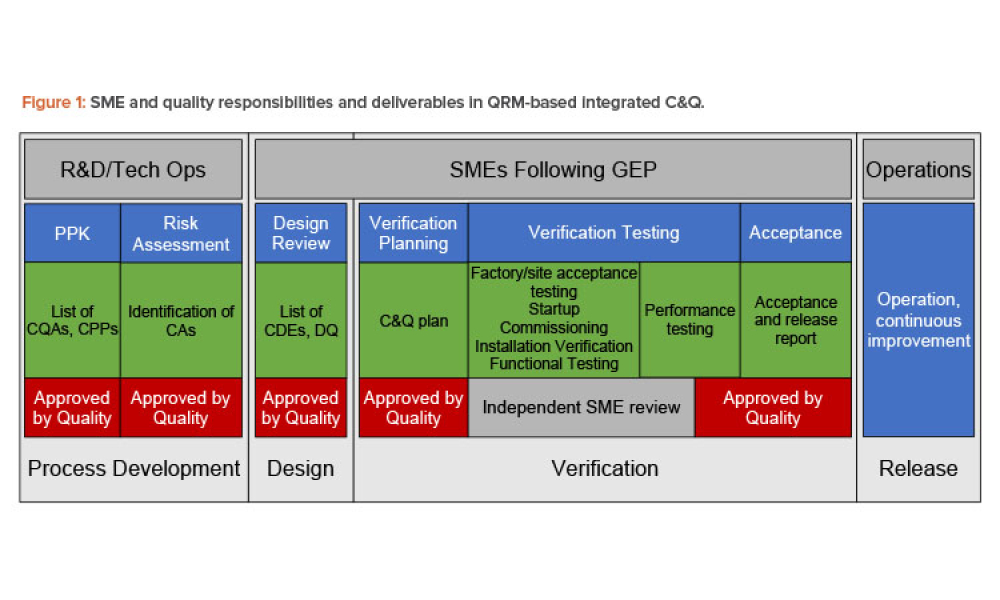
Technical: Good Engineering Practice in Risk-Based Commissioning and Qualification Over the years, the roles and responsibilities of engineering and quality/validation personnel for commissioning and qualification activities have evolved. Now more than ever, C&Q approaches based on quality risk management principles rely heavily on engineering and the application of Good Engineering Practice to provide documentation for the qualification package.
Case Study: Product Development
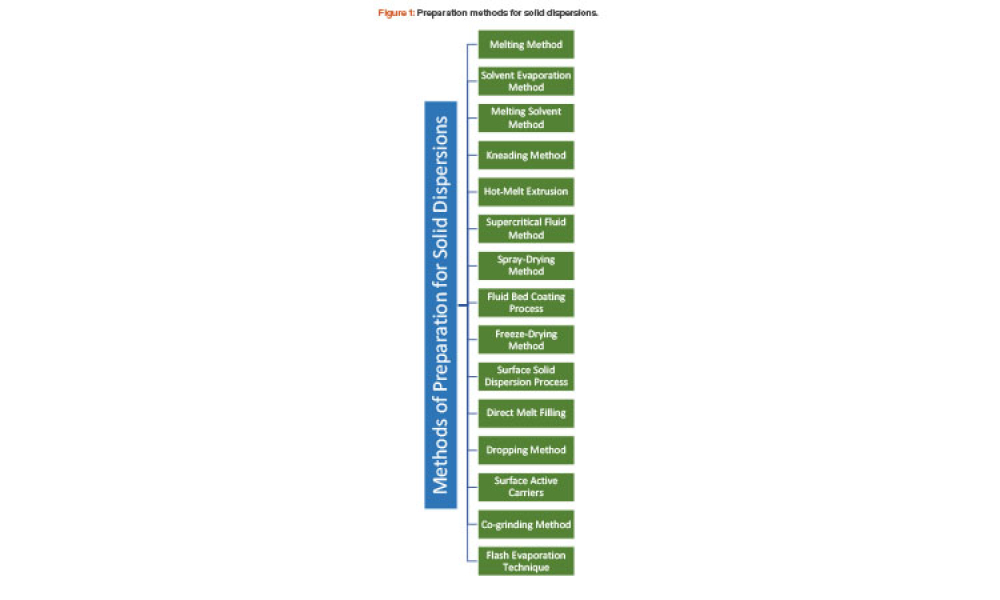
Technical: Solubility Enhancement of Ibuprofen by Porous Solid Dispersion Using a Flash Evaporation Method The solubility behavior of drugs remains one of the most challenging aspects of formulation development and is a key determinant of a drug’s bioavailability. This article describes research aimed to improve solubility of a poorly water-soluble drug (ibuprofen) by preparing a porous solid dispersion using a flash evaporation technique.
In This Issue
This article introduces the concept of robotic process automation (RPA) and discusses how the technology may be used within a GAMP® framework to support both non-GxP and GxP processes.
Brazil’s regulatory authority is working hard to make the nation a larger player in the global pharmaceutical market, and these efforts appear to be working: the life sciences industry has expanded in recent years, and market projections are positive. These ongoing developments represent opportunities for the ISPE Brazil Affiliate as it undergoes its own transformation.
Spring is finally here, signaling a time of new beginnings. Today finds me thinking about International Women’s Day (IWD, observed annually on 8 March), a global day of celebrating the social, economic, cultural, and political achievements of women. This year, International Women’s Day has the theme of equality, a very fitting theme for the topics I want to share with you. This issue’s column...
Did you know that Women in Pharma® (WIP) has launched a program to initiate Mentor Circles around the globe? While great strides have been made to support women in the workplace, women are still significantly underrepresented in upper management in the pharmaceutical industry. The ISPE Women in Pharma® Mentor Circle program...
Annex 2 is the Good Manufacturing Practices (GMP) document by the Pharmaceutical Inspection Co-operation Scheme (PIC/S) addressing manufacture of biological medicinal substances and products for human use. This article shares information about Annex 2 and ISPE’s submitted comments to the draft revision of Annex 2 that was released for public consultation in September 2019.
Virtually every ISPE member has at least one story to tell about how health authority inspections or the review and approval of regulatory applications have affected their efforts to supply critically needed medications to patients globally. Although these stories may emphasize the considerable challenges that ISPE members face, they also frequently identify great opportunities for innovation...
At the 2019 ISPE Global Pharmaceutical Regulatory Summit, regulators updated attendees on approaches to industry innovations and the ongoing work on harmonization and reliance around the...
As the old saying goes, “Time is money.” In today’s industrialized world, this adage is profoundly true. Manufacturers can no longer afford to overlook operational excellence. A new production philosophy called “Lean manufacturing” has been developed to save as much time as possible during manufacturing processes. In some industries, such as the automotive sector, Lean has almost been...
Pam Cheng is Executive Vice President, Global Operations & Information Technology, at AstraZeneca, a United Kingdom–headquartered pharmaceutical company with more than 60,000 employees. In this role, she combines her expertise as an engineer with business savvy and seeks opportunities to lead her company and her industry forward in innovative ways.
Pharmaceutical Engineering® has launched another new section on the Pharmaceutical Engineering Online site: Online Exclusives. Pharmaceutical Engineering Online Exclusives are articles published exclusively on the Pharmaceutical Engineering website. This new feature expands the...
Over the years, the roles and responsibilities of engineering and quality/validation personnel for commissioning and qualification (C&Q) activities have evolved. Now more than ever, commissioning and qualification approaches based on quality risk management (QRM) principles rely heavily on engineering and the application of Good Engineering Practice (GEP) to provide documentation for the...
The solubility behavior of drugs remains one of the most challenging aspects of formulation development and is a key determinant of a drug’s bioavailability. This article describes research aimed to improve solubility of a poorly water-soluble drug (ibuprofen) by preparing a porous solid dispersion using a flash evaporation technique.
Knowledge management (KM) has been a recognized discipline for over 20 years in other industries, although within the biopharmaceutical industry, its discussion and formal implementation have been slow to gain momentum. The topic gained regulatory significance in 2008 when the ICH guidance document Pharmaceutical Quality System: Q10 included knowledge management as a key enabler of a...