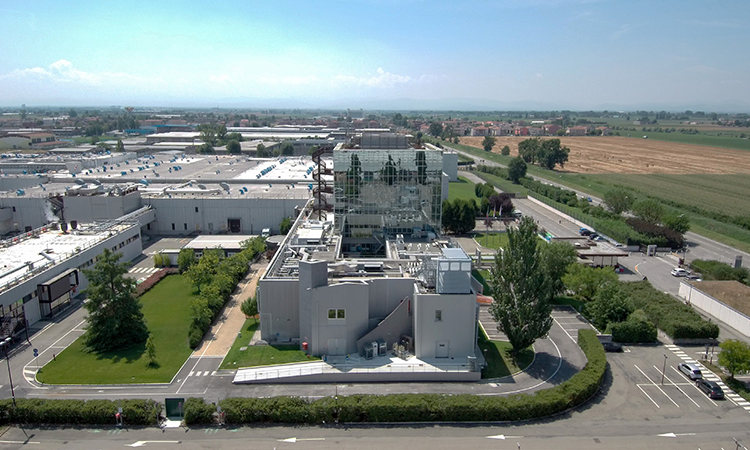
Meet GlaxoSmithKline – 2020 Facility of the Year Award Winner for Social Impact

GlaxoSmithKline is the 2020 Facility of the Year Award Winner for Social Impact for their Attachment Inhibitor (AI) Project in Parma, Italy.

A new award category for 2020, the Social Impact Award recognizes companies who have developed new standards and practices that have prevented drug shortages and increased patients access to medicine, reduced the cost of drug products through the use of new tools or techniques, or accelerated a shift to sustainable facility design which has significantly reduced environmental impact.
GlaxoSmithKline is one of two companies that won the Social Impact Award this year. United Therapeutics is the other.
Project: Attachment Inhibitor (AI) Project
The team used the novel delivery approach of ‘Integrated Project Delivery’ never used before in Italy and were able to build a fully finished facility in 15 months. They paid attention to creating a ‘can do’ attitude and culture whilst implementing accelerated delivery tools. As a result, GSK and ViiV Healthcare ensured the continuity of supply of fostemsavir for clinical trials and allowed the submission of a new drug application to the FDA and CHMP.
FOYA Judges
GlaxoSmithKline (GSK) is a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer. GSK’s HIV research and medication is managed through ViiV Healthcare, a global specialist HIV company focused on advancing science into HIV treatment, prevention, and care. When GSK partnered with ViiV Healthcare to develop a newly acquired investigational HIV product, one of the first things they needed to do was develop a new facility to handle production of the first-in-class HIV treatment. Since the drug is typically used by patients with previous viral failures, who have limited or no treatment options remaining, time was of the essence. GSK met the challenge and in just 15 months, the project team constructed and commissioned a new greenfield NPI facility for high containment.
The accelerated schedule drove the need for a highly integrated program including concurrent design, construction, and commissioning. For the first time in the Italian industry an Integrated Project Delivery approach was used. Designers, contractors, and suppliers from diverse backgrounds and experiences worked together to capitalize on the talents and insights of everyone on the team. The GSK team reduced the overall design time by overlapping the design, procurement, and construction phases thus changing the inherently iterative nature of the design process. Interactive and decision-making workshops were held to evaluate different layout, facility, and process manufacturing options.
The team used 3D Building Information Modeling (BIM) from the early stages of design which proved to be extremely effective and offered several advantages. At the beginning of the design phase, the 3D model helped stakeholders to visualize the design and specify requirements more accurately. During the bidding phase, the model gave construction bidders a clearer overview of the scope of the work. It also helped identify any constructability issues which resulted in significant monetary and time savings. Once construction began, the building was divided into 3 separate construction zones each operating simultaneously.
Due to constraints linked with compliance requirements and the technical/quality risks associated with the commissioning and qualification (C&Q) stages of the project, finding ways to accelerate it was difficult. However, four key factors kept C&Q aligned with the pace of the project.
- A C&Q schedule analysis was performed early on to identify which validation activities were on the project critical path
- Site Acceptance Testing (SAT) was leveraged into IQ/OQ protocols to avoid test repetition
- Every process engineer was supported by a dedicated validation engineer and a computer system engineer to balance workload and enhance technical collaboration
- The extensive participation of production, maintenance, and EHS personnel in FAT and SAT activities helped identify issues early on
“In December 2019, we filed for FDA approval for fostemsavir, after our fastest ever project build,” said Mike Mungall, GSK, Vice President, Global Capital Projects. “The project team, the Parma site, and everyone who had a part in making this happen, is proud to be involved in developing a new treatment that could help people who are living with HIV, but are not able to suppress their virus with other medicines and who could be left with few or no treatments available."
ISPE congratulates GlaxoSmithKline for their FOYA award-winning entry for Social Impact. Learn more about the 2020 FOYA Social Impact winner.
Has your company recently designed, built or renovated a state-of-the-art pharmaceutical or biotechnology facility that is best in its class?
ISPE is now accepting entries into the 2021 ISPE Facility of the Year Awards (FOYA) Program, and your facility may be eligible to apply. Learn more about the Facility of the Year Awards submission process and your company could be the next Facility of the Year Awards Category winner. Nominate your facility in 2021
Want to learn more about the 2020 FOYA winning projects? Get inspiration for future innovations at these complimentary digital events.
Get a free Facility of the Year Awards Day Pass to take an in-depth look at each of the 2020 FOYA winning projects during the 2020 ISPE Annual Meeting & Expo. Claim your complimentary pass today to benefit from 3 hours of compelling and insightful sessions.
Join ISPE and prominent industry leaders on Tuesday, 3 November, as we come together to recognize the outstanding achievements of all the FOYA winners during the 2020 Facility of the Year Awards Virtual Banquet.
%20to%20finished%20facility%20-%203.jpg)