This article explores life-cycle activities for machine learning (ML) within regulated life sciences. It positions and contextualizes the life cycle and management of the machine learning subsystem or components within a wider system life cycle. It also gives general descriptions and guidance illustrated by a case study demonstrating a machine learning application to medical image recognition,...

Downloads
Applying GAMP® Concepts to Machine Learning
Cover: This article explores life-cycle activities for machine learning (ML) within regulated life sciences. It positions and contextualizes the life cycle and management of the machine learning subsystem or components within a wider system life cycle. It also gives general descriptions and guidance illustrated by a case study demonstrating a machine learning application to medical image recognition, or software as a medical device (SaMD).
The Road to Explainable AI in GXP-Regulated Areas
Feature: Recent advances in artificial intelligence (AI) have led to its widespread industrial adoption, with machine learning (ML) algorithms demonstrating advances in performance in a wide range of tasks. However, this comes with an ever-increasing complexity of the algorithms used, rendering such systems more difficult to explain. AI developments offer a solution: Explainable AI (xAI): i.e., additional modules on top of the AI core solution that are designed to explain the results to a human audience.
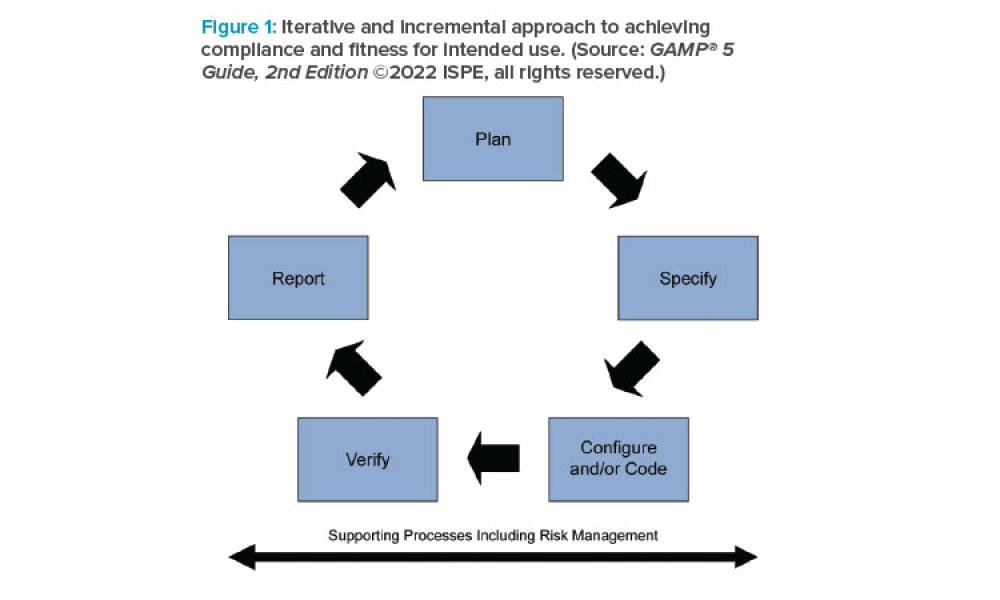
What You Need to Know About GAMP® 5 Guide, 2nd Edition
Feature: ISPE’s GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) (GAMP® 5 Guide, 2nd Edition) maintains the principles and framework of the first edition and updates their application in the modern world, including the increased importance of service providers, evolving approaches to software development, and expanded use of software tools and automation. The 2nd Edition highlights the use of critical thinking by knowledgeable and experienced subject matter experts (SMEs) to define appropriate approaches.
Rapid Filter or Resin Change Strategies for Biomanufacturing
Technical: Pandemic-related supply chain shortages have placed constraints on the supply of essential filters and chromatography resins. An agile regulatory pathway to implement alternative filters and resins into manufacturing is necessary to ensure the continued supply of approved biologics. To allow this in the US and potentially globally, the regulatory strategy proposed in this article is to provide an appropriate characterization package to demonstrate that the alternative filter or resin has a low risk to impact product quality in a prior approval supplement (PAS), and later provide at-scale data as part of an annual report or submission at the time of distribution.
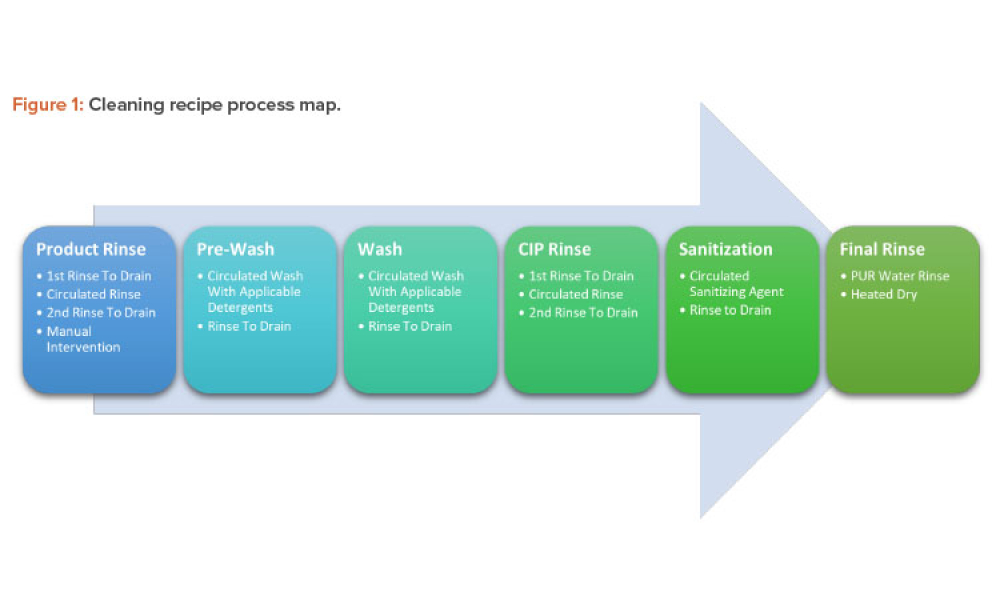
Life-Cycle Approach to Cleaning Topical Drug Products
Technical: Topical drug products and cosmetics are often manufactured in the same facility under a unified quality standard that supports the topical drug products’ performance and label claims. Cleaning is an important component of a manufacturing process, and the process life-cycle approach should be followed for cleaning validation.1 ,2 This article explores the life-cycle approach to cleaning topical drugs and cosmetics with attention to the cleaning design phase and leveraging this information, including lab studies and pilot runs, for qualifying and monitoring the cleaning process.
- 1US Food and Drug Administration. US Code Title 21: Food and Drugs. Chapter 9: Federal Food, Drug, and Cosmetics Act. Subchapter VI: Cosmetics. Section 361: Adulterated Cosmetics. January 2012.
- 2US Food and Drug Administration. “Validation of Cleaning Processes (7/93): Guide to Inspections Validation of Cleaning Processes.” August 2014. https://www.fda.gov/validation-cleaning-processes-793
In This Issue
As we head into 2023, it is hard to believe that it has been four years since the COVID-19 pandemic started to impact our lives and transition us to a new normal. It is also amazing how much technology and innovation continued to advance in those four years to allow our industry to develop new vaccines and innovative products to meet the world’s challenges.
When I started working in the pharmaceutical industry, I was looking for ways to grow my knowledge, my community, and my skillset. When I founded the Emerging Leaders committee within the ISPE D/A/CH (Germany, Austria, and Switzerland) Affiliate, I knew I had found that way.
As a woman of science, I can confidently say that I believe in magic. I believe in the magic that spurs from innovation rooted in desperation, necessity, and determination.
The 2022–2023 ISPE International Board took their places and the gavel passed to a new Chair during the 2022 ISPE Membership Meeting and Awards Lunch on 1 November during the 2022 ISPE Annual Meeting & Expo...
The Commissioning and Qualification (C&Q) CoP was one of the first Communities of Practices to be established at ISPE. One of its founding members, Steve Wisniewski, Principal Consultant, CAI, said the Communities of Practice was formed in 2004 to identify and promote more efficient approaches that resulted in pharmaceutical facilities being fit for their intended purpose.
ISPE’s new Advancing Pharmaceutical Quality (APQ) Guide: Process Performance and Product Quality Monitoring System (PPPQMS) focuses on the key aspects of maintaining and establishing an effective PPPQMS. An effective PPPQMS is crucial to establishing and maintaining a state of control. It enables continual improvement and is key to proactively identifying the need for product quality and...
ISPE’s GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) (GAMP® 5 Guide, 2nd Edition) maintains the principles and framework of the first edition and updates their application in the modern world, including the increased importance of service providers, evolving approaches to software development, and expanded use of software tools and...
In this article, potential Pharma 4.0™ technological solutions that can enhance continuous process verification (CPV) 4.0 are discussed. The necessary paradigm shift will allow companies to predict deviations more accurately, perform root cause analysis (RCA), ensure data integrity and GxP compliance, and ultimately be more competitive in a highly regulated industry.
Pandemic-related supply chain shortages have placed constraints on the supply of essential filters and chromatography resins. An agile regulatory pathway to implement alternative filters and resins into manufacturing is necessary to ensure the continued supply of approved biologics. To allow this in the US and potentially globally, the regulatory strategy proposed in this article is to provide...
Topical drug products and cosmetics are often manufactured in the same facility under a unified quality standard that supports the topical drug products’ performance and label claims. Cleaning is an important component of a manufacturing process, and the process life-cycle approach should be followed for cleaning validation.1
- 1US Food and Drug Administration. US Code Title 21: Food...
Recent advances in artificial intelligence (AI) have led to its widespread industrial adoption, with machine learning (ML) algorithms demonstrating advances in performance in a wide range of tasks. However, this comes with an ever-increasing complexity of the algorithms used, rendering such systems more difficult to explain.1
- 1Samek, W., and K. R. Müller. “Towards Explainable...