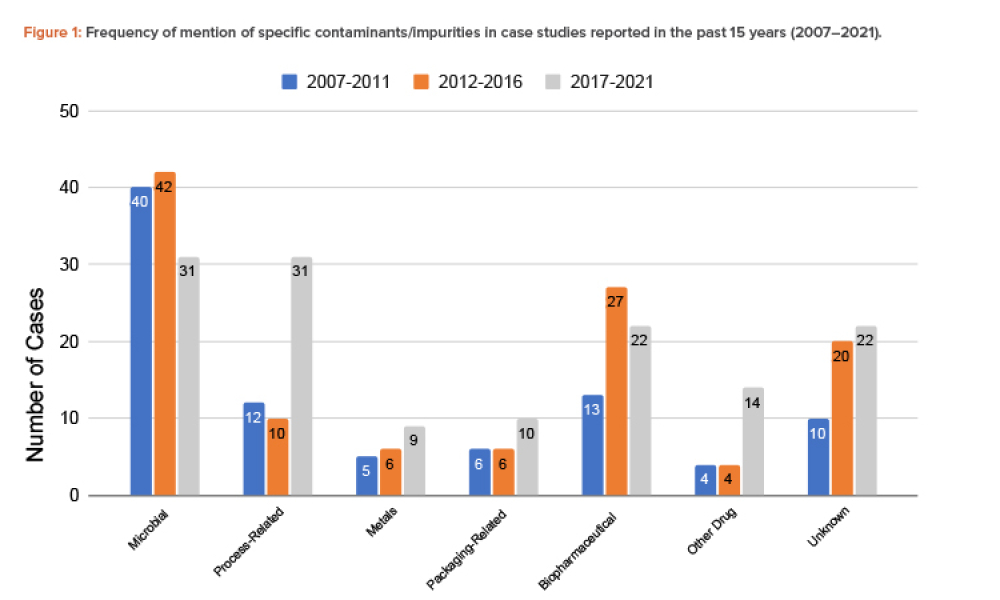
As the pharmaceutical industry faces ever-changing global challenges and market forces, it must review and revise product design to ensure that quality products remain available in the marketplace while moving toward zero pollution for air, water, and soil. This article provides an introduction on how quality products can integrate sustainability by design.

Downloads
Sustainability by Design for Pharmaceutical Products
Cover: As the pharmaceutical industry faces ever-changing global challenges and market forces, it must review and revise product design to ensure that quality products remain available in the marketplace while moving toward zero pollution for air, water, and soil. This article provides an introduction on how quality products can integrate sustainability by design.
Challenges for Net Zero Carbon Pharmaceutical Manufacturing
Feature: Many organizations in the pharmaceutical industry have set net zero carbon goals and targets; they participate in the science-based targets initiative or sustainable markets initiative and disclose carbon emissions in databases like the Carbon Disclosure Project. The vast majority of those in the pharmaceutical industry have shared partial decarbonization plans, but do not yet have concrete plans to achieve these decarbonization goals in the next 10–15 years, often citing a highly regulated environment as a hurdle. Growing public awareness and pressure, as well as technological advances coupled with CO2 prices, are slowly changing the focus, putting the needs of our planet on the agenda.
Sustainability: Corporate Ambition, Governance, & Accelerated Delivery
Feature: The imperative for global action to tackle climate change is clear and the pharmaceutical industry has a key role to play. Governments have entered into international commitments to reduce climate impact (carbon emissions) and protect nature (water, land, air, and biodiversity) with policy frameworks established to facilitate and drive progress against agreed targets. The effect to the pharmaceutical industry spans its end-to-end activities, including the residual impact of used and unused medicines on the environment. Research and development, manufacturing, commercial (sales and marketing) activities, and their extended supply chains including logistics are all within this scope.
Case Study: Meeting Manufacturing Needs with a Net Zero Energy Facility
Case Study: The expected FDA approval for a Treprostinil dry powder inhaler revealed a need for the manufacturer to expand its warehousing and logistics capabilities to support its growing operations. The company’s senior leadership wanted to ensure this expansion came with as minimal an impact on the environment as possible, so a key priority was to provide a net zero energy facility. With a vision for what the project could be, the team named the upcoming endeavor Project Lightyear. To infinity and beyond, indeed.
Cleanroom Recovery Study: Using Computational Fluid Dynamics Methodology
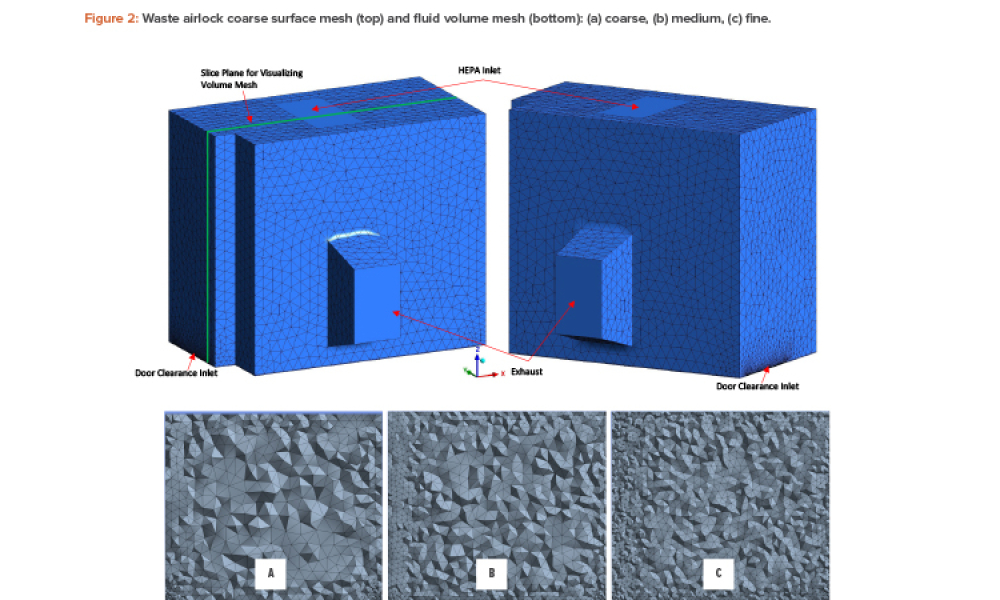
Technical: Computational fluid dynamics (CFD) can reduce or eliminate the uncertainty associated with a cleanroom facility as the planned design can be simulated to predict performance to a high degree of accuracy. This article discusses the use of CFD for the purpose of predicting and optimizing the performance of a cleanroom facility in terms of steady-state airborne particulate levels and for estimating the recovery time to a particulate challenge per ISO 14644-3.
In This Issue
Arriving in the United States at the age of 17 to pursue my dreams was one of the greatest challenges of my life. It was through this experience that I learned the importance of challenging my perspective. This was made possible through my involvement with ISPE, and four years later, I’m proud to announce the launch of Mentor ISPE.
When nominating Martin (Marty) Lipa, Executive Director, Knowledge Management, Merck & Co. Inc., for the 2022 Max Seales Yonker Member of the Year Award, Anne Greene, Professor, Technological University Dublin, said, “Arguably the last year has been pivotal for the practice of knowledge management (KM) in the pharmaceutical industry based on new knowledge management frameworks and guidance...
With this issue, Pharmaceutical Engineering® launches a new feature in P+E: profiles of Communities of Practice (CoP) leaders. These leaders are central to the success of ISPE’s CoPs, which spearhead the generation of ISPE’s gold standard content, including Good Practice Guides, Pharmaceutical Engineering articles, conference presentations, and training programs.
With this issue, Pharmaceutical Engineering® launches a new feature in P+E: profiles of Communities of Practice (CoP) leaders. These leaders are central to the success of ISPE’s CoPs, which spearhead the generation of ISPE’s gold standard content, including Good Practice Guides, Pharmaceutical Engineering articles, conference presentations, and training programs.
Cultural excellence is the expressed and implied ways in which an organization operates. Excellence in organizational culture is essential for delivering robust and sustained quality performance and ensuring patient-focused outcomes. ISPE’s new Advancing Pharmaceutical Quality (APQ) Guide: Cultural Excellence provides a quality management framework for assessing and advancing an organization’s...
The imperative for global action to tackle climate change is clear and the pharmaceutical industry has a key role to play. Governments have entered into international commitments to reduce climate impact (carbon emissions) and protect nature (water, land, air, and biodiversity) with policy frameworks established to facilitate and drive progress against agreed targets.1
- 1United...
The expected FDA approval for a Treprostinil dry powder inhaler revealed a need for the manufacturer to expand its warehousing and logistics capabilities to support its growing operations. The company’s senior leadership wanted to ensure this expansion came with as minimal an impact on the environment as possible, so a key priority was to provide a net zero energy facility. With a vision for...
Computational fluid dynamics (CFD) can reduce or eliminate the uncertainty associated with a cleanroom facility as the planned design can be simulated to predict performance to a high degree of accuracy. This article discusses the use of CFD for the purpose of predicting and optimizing the performance of a cleanroom facility in terms of steady-state airborne particulate levels and for...
Many emerging tools and technologies support the environmental sustainability of the pharmaceutical industry. In facility design, solutions are derived through science-based analyses of environmental impacts from the materials, processes, services, and architecture. From greener energy sources to a reduction in the type and amount of emissions, we are seeing improvement in this sector’s...
While financial investment in novel therapies provides patients with new treatment options and improved quality of care, the pharmaceutical industry also recognizes its responsibility to transition toward more sustainable development, manufacturing, and stewardship of medicines throughout their life cycle.
To enable changes across the pharmaceutical industry, sustainability should be included alongside quality, efficacy, and safety when assessing medicines. This article reviews two case studies that cover sustainable pack types and extension of shelf life. With the drive to manage unmet medical need through acceleration of drug development programs, postapproval sustainability variations will...
2023 continues to move right along—the first quarter is almost over. I remain very optimistic about this year as ISPE International, with support from the International Board, continues to make progress on our 2023 objectives and the 2023—2025 ISPE Strategic Plan.
Sustainability is a buzzword that has been tossed back and forth across industries for decades. For a while, it was seen as something that consumers didn’t really care about, but now the reality is different. According to a 2022 study, nearly 80% of consumers think about sustainability when purchasing products. Sustainability efforts can range from a specific product to an entire brand,...