About the Author
Related Articles
In the pharmaceutical industry, which is highly regulated, aseptic processing is a critical component that ensures the sterility of products. Regulators have a set of comprehensive requirements that minimize the risk of contamination. Regulators set the requirements; however, the industry has an obligation to the patients who rely on and expect a drug that is safe and free of contamination....
Like any investment in digital manufacturing systems, demonstrating business payback before and after investment is critical to getting MES projects approved and verifying profitability after investment. Whether it is introducing MES, expanding it to additional sites, switching software, upgrading existing software, or developing electronic production records (EPR) for new drug products,...
Sustainability is emerging as a pivotal force influencing the pharmaceutical industry’s dynamics and steering companies towards transformative change. The focus of this analysis is to delve into the sustainability key performance indicators (KPIs) of prominent pharmaceutical players, namely Pfizer, Sanofi, Glaxo Smith Kline (GSK), Becton Dickinson, Johnson & Johnson, Abbott, Eli Lilly, and...

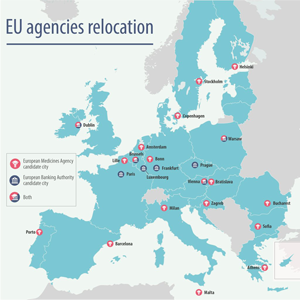 The European Commission has chosen Amsterdam as the new host of the European Medicines Agency (EMA) headquarters. The city was awarded the coveted prize after it won a coin toss with Milan, which received the same number of votes after three rounds of voting by the 27 European Union (EU) foreign ministers.
The European Commission has chosen Amsterdam as the new host of the European Medicines Agency (EMA) headquarters. The city was awarded the coveted prize after it won a coin toss with Milan, which received the same number of votes after three rounds of voting by the 27 European Union (EU) foreign ministers.

