FDA's Revised Draft Guidance on Quality Metrics Has Been Issued

FDA Releases Revised Draft Guidance on Quality Metrics and ISPE Announces 2017 Conference on Quality Culture
FDA Issues Revised Draft Guidance on Quality Metrics
The US Food and Drug Administration (FDA) released on 23 November 2016 a much-anticipated revision of its draft guidance on the collection of quality metrics. The revised Submission of Quality Metrics Data Guidance for Industry is a response to both ISPE and industry concerns that the original guidance was too demanding. In the document, FDA lists the revisions: "The revised draft guidance includes the following changes from the earlier draft guidance: Adoption of a phased-in (voluntary) approach, reduction in the number of data elements requested (i.e., reduction in reporting burden), support for both product reports and site reports, modifications to the quality metrics data definitions, addition of clarifying examples for the definitions, addition of comment fields, and clarification of special considerations for non-application and OTC [over-the-counter] product reporting." Read the revised Submission of Quality Metrics Data Guidance for Industry here, and listen to the FDA webinar here.
ISPE 2017 Conference on Quality Culture
The 2017 ISPE Conference on Excellence in Quality Culture and Performance to be held in Bethesda, Maryland, 25 – 26 April 2017. The conference will coincide with the publication of the ISPE Cultural Excellence report, a collection of practical powerful tools that outlines a comprehensive behavior-based approach to improving quality culture as a means of delivering enhanced quality outcomes. Conference attendees will learn from industry peers through case studies and the sharing of best practice:
- How to implement the practical approaches and tools compiled in the Cultural Excellence report
- How industry leaders can contribute to and help shape quality culture
- Which best practices enable a collective mindset to drive toward improving quality
- Gemba’s key role in coaching and mentoring the desired attitudes and behaviors
- How to use a practical new tool to target and measure behavior that matter
- Which best practices are required for effective management oversight and review
- What critical enablers are necessary to build and sustain a culture of excellence.
Read the Quarterly report on Quality Culture in the November/December 2016 issue of Pharmaceutical Engineering®Magazine.
Quality Culture in Action at ISPE 2016 Annual Meeting & Expo
At a well-attended Quality Metrics session at the ISPE Annual Meeting & Expo in Atlanta on 21 September there were excellent, thought-provoking and informative presentations followed by a lively Q&A discussion. Understanding quality metrics applied to assess quality performance and the underpinning importance of a quality culture were themes of the session. Presenters were: Marie Mathews, Compliance Officer, CDER/OC/OMQ, FDA; and Dr. Nuala Calnan, Research Fellow, Dublin Institute of Technology, Ireland. In a separate, related session, Mairead Goetz, Global Head Analytical Science and Technology, Novartis provided a company perspective.
Counting the Hard to Count
Mathews presented an FDA field perspective on quality metrics and culture. Building upon the oft-cited quotation, “not everything that can be counted counts, and not everything that counts can be counted”, she reflected that a traditional approach to quality metrics may capture OOS, deviations, trends, rejects, complaints and recalls amongst others, however, the values can be rendered useless unless the correct information is used and appropriate action taken promptly. “Some of the more out of control companies I’ve seen look pretty good with these metrics, on first glance” she said. It may not be until the FDA or another agency conducts an inspection that gaps are revealed. By then, the company has already lost control and extraordinary amounts of effort are required to get back on track. She stated that if OOS results are improperly invalidated and not tracked, then trends, reworks, rejects and recalls may not be captured. When difficult decisions need to be made, what path to choose? If an OOS result is observed, are other lots examined for possible impact? Mathews gave the example of a batch rejection due to foreign particles in an API where the extent of the problem was insufficiently investigated, and an inappropriately long period passed before a project was initiated to determine root cause and take corrective action. Many companies are good at creating mission or value statements but not as capable of assessing their culture. She quoted an example of a very out of control plant which had a laboratory with 100% turnover of staff in two years. The plant had not recognized a huge red flag. Mathews then cited four misconceptions about quality culture:
- If an employee sees something objectionable, they will let someone know
- We have an internal whistle-blower line, so employees will use this
- We would know if one of our employees contacts FDA
- If I find out about a problem, it makes me personally responsible
What does works, she added, are actions such as conducting a survey of all employees on culture, with guaranteed anonymity; and a review of employee incentives, both monetary and non-monetary, to encourage wanted behaviors and discourage unwanted behaviors. She closed by addressing the issues of empowerment and transparency as quality culture enablers.
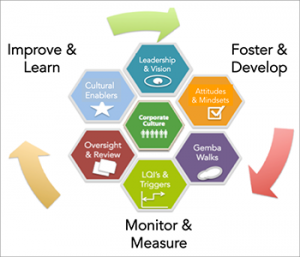
ISPE Cultural Excellence During two years of collaboration, 35 members from 28 companies in six sub teams are producing a series of deliverables, which will complete by year-end of 2016. Each sub team is working on one of the dimensions in the outer circle of the diagram.
Dr. Calnan gave an update on the ISPE Cultural Excellence Program. She explained that the program was aimed at promoting, coaching and leading specific desired behaviors while identifying and preventing specific undesired behaviors. The six dimensions of the framework were described as shown in the diagram. Dr. Calnan described these deliverables as tools for use by companies, sites and individuals to move towards cultural excellence. Read Dr. Calnan’s articles on cultural excellence in Pharmaceutical Engineering®Magazine.
A Journey of Quality Culture and Performance: A Novartis Perspective
Mairead Goetz described the challenge Novartis faced with several acquisitions, different business models, cultures and standards across 67 plants employing greater than 6,600 people. The goal is to evolve a strong, consistent and sustainable quality culture throughout Novartis with sites having ownership and commitment top down. There are 15 basic culture actions, which inform specifically tailored site change plans. Even within a large organization committed to quality improvement, each site has its own culture, requiring specific initiatives to improve their individual culture maturity. Progress is assessed using a bi-annual company-wide survey with 12 questions, summarized using a scorecard matrix of survey results, KPIs and KQIs. To approach these goals Novartis has one quality standard for the whole network, uses key performance indicators constantly and has a strong foundation of values and behaviors sponsored by senior management. Many examples were given although Goetz stressed that movement of quality performance requires more than just numbers.
Quality culture change is a long journey and requires perseverance. Sites tend to initially overestimate their quality maturity, which unto itself is a subjective assessment. Management has the critical responsibility to foster and enable the change journey and the ongoing surveys are an extremely important tool to provide visibility to each site’s progress.

