2018 ISPE Singapore Conference Highlights

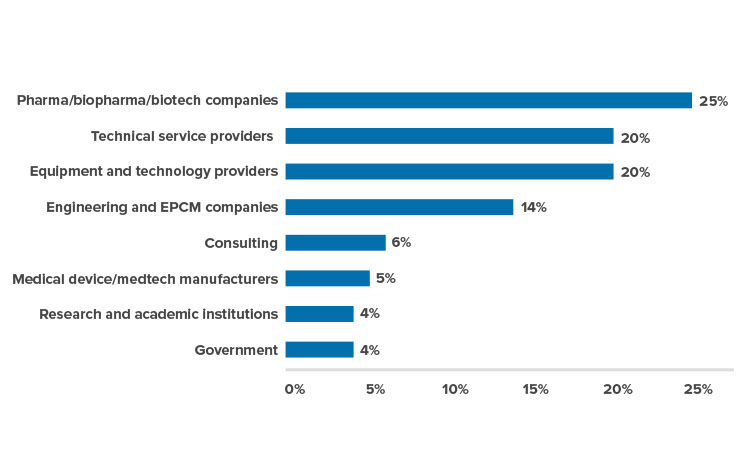
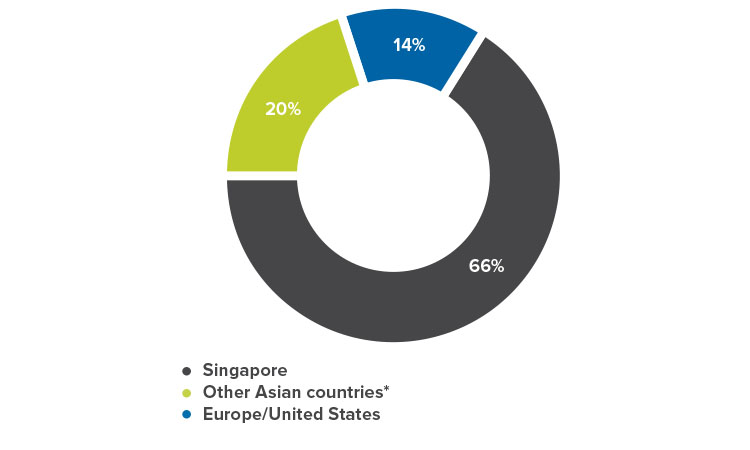
Record-setting 1,000 participants from 25 countries
The 2018 ISPE Singapore Conference and Exhibition, 30–31 August, drew over 1,000 participants, a record attendance for the 18 years of the conference. Attendees included Southeast Asian delegations from Indonesia, Thailand, and Vietnam. Over 65 speakers shared thought leadership, best practices, and real-life experiences. The event also featured the region’s first Women in Pharma® panel session, held in conjunction with the conference.

Opening Plenary
Ferry Soetikno, Chief Executive Officer, Dexa Group, Indonesia, shared his insights on ensuring the supply of quality medications beyond domestic markets. With its patient- and quality-centric ethos, Dexa’s vision and achievements have extended beyond Indonesia. The company’s products include nonsterile oral dosage forms, sterile injectables, topicals, OTC and herbal preparations, and consumer products. The company’s products are marketed in Asia, Africa, and the Pacific. It has two cGMP facilities, one of which has been approved by the MHRA (UK), TGA (Australia), and Darmstadt.
ISPE 2017–2018 Board Chair Tim Howard pointed toward strategic plans, which focus on globalization and regulatory harmonization. Drug shortages will require continuous vigilance, aided by regulatory and industry efforts on quality metrics and quality culture. He announced an upcoming APAC regional conference for 2019.
The importance of quality and culture was underlined by the plenary panel discussion: “Driving a Quality Culture through Leadership,” moderated by Conference Chair Pierre Winnepenninckx, CEO and Founder, No Deviation Pte, Ltd., and led by Chong Meng Chai, Head of Mammalian Manufacturing, Lonza Biologics, Singapore; Vincent Loret, Site Director, GlaxoSmithKline, Singapore; and Dr. Jincai Li, Vice President, Drug Substance Manufacturing (MFG1), WuXi Biologics, China.
Chai cautioned the audience not to assume that established systems and workflows are always robust. He stressed the importance of leaders staying connected to the shop floor to reduce the gap between work as they imagine it and work as it actually happens.
This was supported by Loret, who emphasized the need to “walk the talk.” He said this drive should come from leadership and not external facilitators.
The panel agreed that key performance indicators (KPIs) should drive positive behaviors and solutions, but warned against using KPIs to reduce deviations, since that could lead to adverse behaviors such as hiding deviations and cosmetic corrective and preventive actions. Applied correctly, KPIs enable tracking and help transform “non-right-first-time” incidents into improvements.
While all the panelists said they do a Gemba walk once a week, they wished to do so more often because of the energy it gives people and the encouragement it provides to identify problems and propose solutions. The session concluded with a discussion on the critical importance of trust, openness, and transparency by demonstrating the positive consequences and improvements they produce.
Sterile and Aseptic Operations
Maurice Parlane, ISPE Australasia Board Director, Centre for Biopharmaceutical Excellence Director, and Principal/Director of NewWayz Consulting Ltd., New Zealand, spoke on risk assessment (RA) in aseptic processing. The typical linear 1–5 scale used in the failure modes and e¬ffects analysis may not be the best representation of risk ranking, he said. There are many factors to consider and a reality check with the monitoring system should be done.
Since RA focuses on the probability of occurrence, does a ranking of 4 (very probable) mean it is two times more likely to happen than a risk ranking of 2? A logarithmic or weighted scale could be a better approach.
Process Validation
Hazem Eleskandarani, Global Director, Commissioning & Qualification, Johnson & Johnson, USA, explained that process validation (PV) is demonstrated through design. PV and commissioning and qualification (C&Q) are not two separate steps but are integral parts of a continuous process, known as commissioning qualification validation (CQV). This aligns with the second edition of the ISPE Baseline® Guide Volume 5: Commissioning and Qualification, currently in development (publication expected in 2019). CQV should not wholly be the quality team’s responsibility but should also fall to subject matter experts.
Eleskandarani also discussed the critical design elements of an integrated CQV process:
- Trained personnel working on the end-to-end process
- Execution plan or road map
- Follow regulatory guides
- Work with the quality team throughout (not only at the end)
- Know and adhere to policies and procedures
- Use the right materials and resources
- Follow proper C&Q of facility and equipment for a smooth PV
- Create accurate and timely documentation
Maurice Parlane also moderated the panel discussion “Transitioning from Project to Validation” with panelists Paul Si, Head Project Management Asia, Novartis, Singapore; Melis Tay, Operations Start Up Manager, AbbVie, Singapore; and Michael Lee, Senior Vice President, Mab Venture Bio Company, China. All agreed on the importance of the 3Ps: project, process, and product. Eleskandarani added that clearly defining the objectives from the start and making sure the whole team is aware of the objectives give a sense of ownership, helping both teams and individuals integrate them into their own objectives.
In the case of how involved the project management should be in process validation, both Lee and Eleskandarani agreed that the handoff from project to operations varies with project and should be a gradual process. Si said that a part of the operations team could be integrated into the C&Q team at identified points during the C&Q time frame for recipe testing done by the operations team. Lee emphasized that the users (operations) are the clients and the project management team should take their input into account and not shrug off their comments.
With deviations, Si noted that predefining the stage at which it is a punch-list item and at which it is a deviation would save time and allow the team to be more focused on solving the problem. Eleskandarani said that knowing the objectives, using factory acceptance tests to draft SOPs, and doing test runs with the equipment and automation systems, among other actions, would smooth the process and increase people’s knowledge and expertise. “Practice makes perfect,” he said.
Logistics and Distribution
Participants in this track agreed that as more regions, including Asia, adopt and require serialization, its key benefits and enormous potential of end-to-end tracing—even to patient level—with fully attributable information will become apparent. Serialization will also expedite recalls, help prevent identify theft and counterfeiting, improve complaint management, and aid deviation investigation.
Main challenges include long timelines for successful implementation, varying standards and systems, and making regulations mandatory. In initial stages complaints may increase due to the need for all parties, such as wholesalers and pharmacies, to be ready.
Blockchain and integrating databases were also discussed.

Women in Pharma®
The first Women in Pharma event organized by the Singapore affiliate was moderated by Shanshan Liu, VP of ISPE Singapore. The panel of female leaders and role models in the pharma industry were: Sook Peng Chua, ASEAN Regulatory and Quality Compliance Director, Johnson & Johnson, Singapore; Christine Moore, Global Head and Executive Director, GRACS CMC–Policy, Merck Sharp & Dohme Corporation, USA; Dr. Vasiliki (Vee) Revithi, former head of EOF/Greece, GMDP Inspectorate, Greece; and Michelle Peake, Senior General Manager, PT Kalbio Global Medika, Indonesia.
They shared their experiences, stories, and aspirations in both careers and personal lives during the interactive session, including insights on planning a successful career path, keys to opportunity, career barriers, and work–life balance. Male audience members also participated actively, gaining awareness of and committing to making the industry more diverse and inclusive.
Single-Use Systems
This track was well balanced between insights from suppliers, manufacturers, and service providers. All emphasized the significance of close collaboration between supplier and end user to implement single-use systems. Sessions presented pros and cons associated with single-use systems, as well as considerations in choosing single-use over traditional stainless steel systems. Aside from saving on capital costs and utility consumption, one benefit of single-use systems is the possibility of “scaling out” to avoid the risks associated with “scaling up.”
Participants also shared the latest technology in buffer systems. While this may sound simple, significant challenges and planning are involved. To simplify the complex in-line-dilution buffer skid, the single-use version is in development.
Issues with leachables, extractables, and absorption are still major concerns for single use. The selection of various materials, how surface/volume ratio varies the impact of bag material, and even quality variations in the same material from different suppliers or even different batches from the same supplier, were addressed.
Dr. Jincai Li, Vice President, Drug Substance Manufacturing (MFG1), WuXi Biologics, China, presented a case study of transferring a stainless steel production line to a single-use production line. The company expects to achieve a DS capacity of 220 kiloliters globally by 2021, reflecting the growing roles of the Asia market and Asian manufacturers in the pharma/bio world.
Regulatory Affairs
Regulatory updates were followed by a discussion moderated by Bob Tribe, former Chairman, PIC/S, Australia, and Asia–Pacific Regulatory A¬ airs Advisor, ISPE. Regulatory members were:
- Dr. Vasiliki Revithi, former Good Manufacturing and Distribution Inspectorate Head, Ethnikos Organismos Farmakon (Greek National Organization for Medicines)
- Meow Hoe Boon, 2018–2019 PIC/S Chair who shared on “GMP Harmonisation & GMP Inspection Reliance from a PIC/S Perspective”
- Hock Sia Chong, Health Sciences Authority, Singapore who presented on “ASEAN MRA on GMP Inspection: Benefits to ASEAN Economic Community”
- Vladimir Orlov, State Institute of Drugs & Good Practices, Russia, who spoke on “Foreign Medicines Inspections in 2017: Overview of Results”
- Dr. Achiraya Praisuwan, Thai FDA who gave “Regulatory Updates: Thai FDA”

Some highlights of the panel discussion were:
- China FDA showed considerable interest in PIC/S and is expected to apply for membership in the next year or so. CDSCO, India, also showed interest in PIC/S, but it was not known whether they would make an application.
- The Russian regulatory authority recently made a pre-accession application for PIC/S membership.
- There was strong support for the PIC/S “Inspection Reliance” initiative, as it will avoid unnecessary duplication of GMP inspection work.
- With the recent publication of version 14 of the PIC/S GMP Guide, it is common practice for PIC/S member authorities to give manufacturers a 12-month transition period to adjust to the new requirements. However, some PIC/S member authorities have been very slow to adopt new versions, with several authorities still using version 8 as their legal requirement.
- Although the ASEAN mutual recognition agreement (MRA) on GMP inspection currently applies only to medicines, it will soon be expanded to include APIs, biologicals, and herbals.
- As of August 2018, the US FDA had to complete the assessments of 14 EU member states by 15 July 2019, per its MRA with the EU. If those assessments are not completed by this date, the MRA will not proceed.




