Patient Perceptions of IMPs Survey

This three-part paper focuses on the final results from the EU and China Patient Perceptions of Investigational Medicinal Products surveys and compares some of these results with the original "ISPE Project Concerning Patient Experience with Clinical Trial Materials" published in 2013.
Contributions and opinions are based on the individuals’ knowledge and expertise; this presentation should not be construed as a statement or opinion by Catalent Pharma Solutions, or any other member of the task team.
Every clinical trial professional faces the same challenge: Get 1) the right product 2) to the right patient 3) at the right time 4) every time. This bar only gets higher when new products with inherent stability challenges (such as biologics or patient-tailored medicines), increasing globalization of clinical trials, and financial constraints are part of the picture. A sound appreciation for the role of clinical site personnel, a commitment to clear instructions for both the patient and the clinical site, and flexibility in IMP design may help address all these concerns. Traditionally, preparation of investigational medicinal products (IMPs) had been focused on upstream activities compliant with current good manufacturing and distribution (cGMP and cGDP) practices, with little thought for the preferences of the end user—the patient or the clinical site. This may be changing, however, as patient centricity and global perspectives increasingly influence decision making to improve patient adherence and compliance to protocol.
Original study
In 2012, ISPE’s Investigational Products Community of Practice (IP CoP) formed a Patient Survey Project Team to conduct what they hoped would be the first industry-sponsored global survey to understand patient experiences with clinical study supplies.1 Published in 2013, the "ISPE Project Concerning Patient Experiences with Clinical Trial Materials" surveyed 1,425 clinical trial patients to learn about the suitability of clinical materials, collect patients’ opinions about their experiences, and gather suggestions for future improvements. While study findings indicated a good level of reported drug compliance and demonstrated that patients were generally satisfied with the IMPs they received, the results also revealed a number of areas for consideration in improving medicine kit design. A significant challenge, however, was that despite the project team’s considerable efforts to recruit a globally diverse study population, almost all (97%) of the respondents were from the United States.
Revised and expanded
To help identify possible geographic differences in patient preferences, regional ISPE Investigational Product (IP) teams decided to adapt and expand the original US-weighted survey to explore detailed feedback from three populations. The first spinoff study was conducted in the United Kingdom (UK) and European Union (EU). In a similar timeframe, the China IP CoP completed a study in China, a region that had never provided feedback on IMPs before. A third arm, conducted in Japan, is still ongoing. Survey questions for each of the studies were adapted from the original 2013 study, and centered on whether IMP kit design, packaging, and labeling influenced patient compliance with the protocol and retention within the study. These are important cost drivers in clinical trials, where noncompliance can lead to patient failure and unevaluable data. Even a small percentage of failures can be detrimental, since each lost patient has been estimated to cost the study sponsor as much as $42,000 per patient, even if they’re not replaced.2 All studies went live in the fall of 2015; as before, all were conducted under ISPE auspices. Interim results for the EU and China studies were presented at the ISPE 2015 Annual Meeting, and were published in Pharmaceutical Engineering in 2016.3 Final results for the EU and China studies were presented at the ISPE 2016 European Conference, ISPE 2016 China Spring Conference, and ISPE 2016 Japan Annual Meeting. This paper focuses on final results from the EU and China surveys and compares some of these results with the original 2013 survey, which for the purposes of this paper will be referred to as the "original US Study," based on the location of most of the respondents. The vast majority of patients in both studies found their medication easy to use; this is no reason for complacency, however
Objectives Patient Perceptions of IMPs Surveys
Research teams sought information that could help ensure patient compliance, adherence, and retention in clinical trials. If these new survey results revealed major geographic differences, they could affect how IMP kits are designed, labeled, and packaged for different regions. These are important concerns in clinical trials, which are frequently conducted in multiple countries, languages, and regions. Goals were to:
- Gather patient feedback on the suitability of clinical materials provided
- Obtain patient suggestions for improvements
- Understand the effect of key patient differentiators
The team hoped to gather results that would support management decisions about IMPs, as well as increase collaboration between global regulatory bodies, sponsor companies engaged in the IMP sector, and facilitator organizations like ISPE so that enlightened global guidance could result.
The vast majority of patients in both studies found their medication easy to use; this is no reason for complacency, however
Methodology
Access to appropriate patient populations was instrumental to survey success, with patient anonymity being carefully controlled. EU The EU team partnered with three agencies who had access to patient groups:
- UK National Health Service (NHS)
- UK National Institute for Health Research (NIHR)
- European Patients Academy on Therapeutic Innovation (EUPATI)
All had read the results of the original US study with interest and all were actively focused on public and patient involvement in clinical research. The study was conducted electronically and in English only, with 48 questions adapted from the original US study. ISPE administered the survey and aggregated the responses; NHS, NIHR, and EUPATI disseminated the surveys to patients through clinical trials pharmacies, research nurses, or patient advocacy groups; the Robertson Centre for Biostatistics, University of Glasgow, analyzed and reported on the resulting data. The final EU analysis contained data on 109 patients out of 543 collected responses. Not all patients responded to every question, thus, charts below show varying Ns for the EU study population. China In China, the ISPE China IP CoP partnered with Drug Information Association China to enlist five site management organizations to collect responses:
- Hangzhou Tigermed Consulting Co., Ltd.
- LinkStart
- Medkey Med-Tech Development Co., Ltd.
- SMO ClinPlus
- WuXi Apptech
The patient survey was also sponsored by seven companies:
- Almac
- Cardinal Health
- Catalent
- Fisher Clinical Services
- Lilly
- Merck & Co., Inc.
- Pfizer
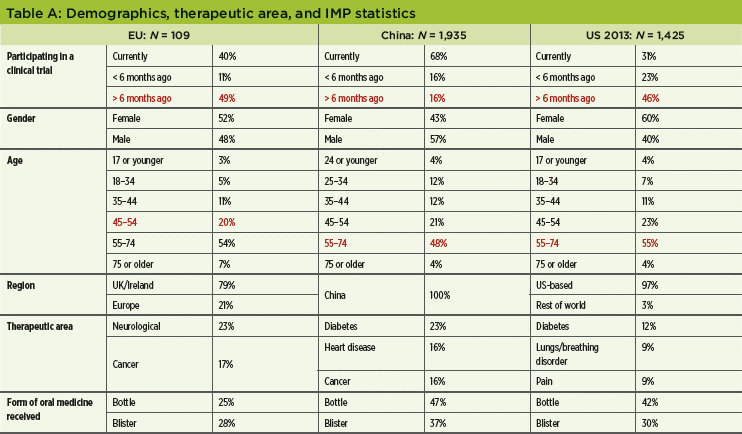
As in the EU, the China survey contained 46 questions modified from the original US study, which were translated into Chinese. Data were collected via mobile or paper versions, depending on the patients’ preferences. Surveys were conducted in person at study sites. Total valid data for analysis was 1,935 out of 2,488 collected responses. Unless indicated otherwise, N is always the total study population. See Table A for study demographics, therapeutic area, and IMP statistics.
- 1International Society for Pharmaceutical Engineering. "Report on the ISPE Project Concerning Patient Experiences with Clinical Trial Materials." November 2013. www.ispe.org/patient-initiative/2013novreport.pdf.
- 2 Pharmaceutical Research and Manufacturers Association and Battelle Technology Partnership Practice. "Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies." March 2015. www.phrma.org/sites/default/files/pdf/biopharmaceuticalindustry-sponsored-clinical-trials-impact-on-state-economies.pdf.
- 3Sadler-Williams, Esther. "Patient Perceptions of IMPs." Pharmaceutical Engineering 36, no. 1 (January/February 2016): 22–23.
Results
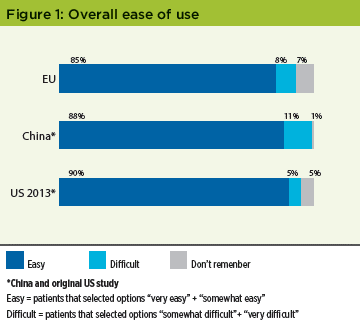
Overall ease of use The vast majority of patients in both studies found their medication easy to use: 85% in the EU study, and 88% in the China arm, which is consistent with the 77% reported in the original US study (Figure 1). This is no reason for complacency, however, as some later results will show.
Kit design supported taking medicine on schedule
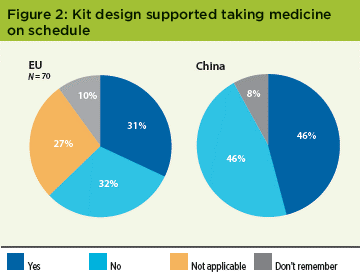
In the EU survey, 32% found kit design important—almost the same number that found it unimportant. The Chinese population also split evenly: 46% said the design was helpful, and 46% said it wasn’t (Figure 2). This unequivocal result in both EU and China was different from the original US study, where 60% said that the kit design helped them take the medication on schedule.
What would help the patient take the medicine on schedule?
To delve deeper into this issue, the patients were asked "What would help you take the medication on schedule?" 81% of EU patients stated clear dosing information on the label (58% US) as useful, followed by 66% who chose the provision of individual dosing units in the medicine container, and 64% who said that verbal instructions from site personnel were useful. In China, 77% cited "instructions from my physician/nurse/pharmacist at every visit" as helpful, followed by 57% who chose medicine kits organized in daily or weekly doses, and 55% who chose dosing information on the label.
Most helpful form of instruction
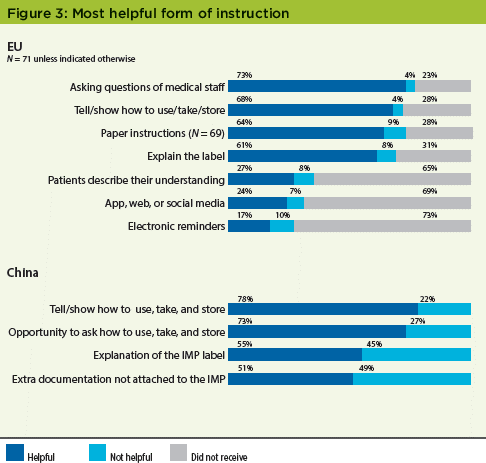
Despite a stated preference for dosing information on kit labels in the EU study, 73% of patients said that being able to question the medical staff was most helpful in understanding dosage, storage, and other adherence criteria. Results from the China study were even stronger: 78% cited "Someone telling or showing you how to take/use/store the clinical trial medicine" as helpful (Figure 3). In the original US study, 77% of patients said that having someone tell or show how to use, take, and store the clinical trial medicine was very helpful; 76% said that having the opportunity to ask questions on how to take, use, and store the medicine was very helpful.
Medicine form preference
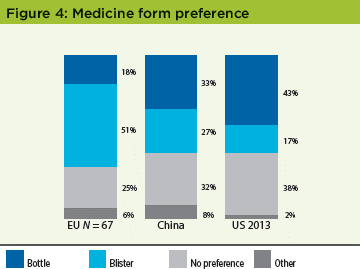
The EU study population showed a strong preference for blister packs (51%). In China, a very small plurality (33%) favored bottles, followed by 27% that preferred blister packs. Overall, these results demonstrated regional differences between the EU, China, and the United States in the preferred presentation for oral medication. This preference for bottles in China was not as strong as in the original US study: 43% bottles vs. 17% for blisters (Figure 4).
Size, storage, and transportation
In the EU survey, 83% of patients said their IMP kit was very easy or OK to store; 90% of patients in the China study said their medicine was easy to store. These results are similar to the original US study, where 82% of patients said their medicine kits were easy or somewhat easy to store. At the outset this was a feature that the survey team was certain would be a concern to patients; the overall consistency and level of patient satisfaction is reassuring. When asked if the medicine kit size was easy to transport, the answers were similar: 72% of EU patients found the kit was just the right size, compared to 73% from patients in China, and 77% from US patients.
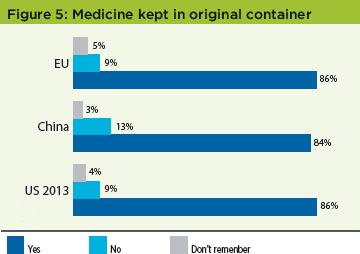
Medicine kept in original container
A concern to the industry as a whole is that patients may remove their medication from the clinical trial kit provided, thus risking incorrect dosing. However, patients in all studies reported similarly encouraging responses: 86% of EU patients and 84% of China patients kept their medicines in the original bottles. This corresponds well with the 86% of US patients who did similarly (Figure 5).
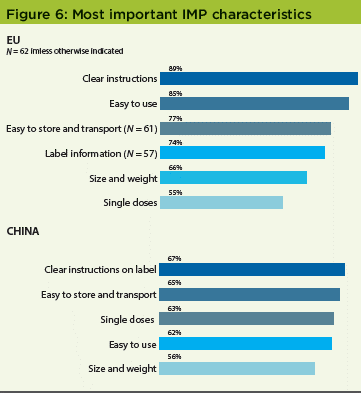
Most important IMP characteristics
Patients overwhelmingly rated clear instructions and ease of use as the most important characteristics of their IMP kits: 89% and 85%, respectively, in the original US study, and 67% and 62%, respectively, in the China survey (Figure 6). In the US survey, 69% of patients rated clear instructions as most important; 64% cited ease of use.
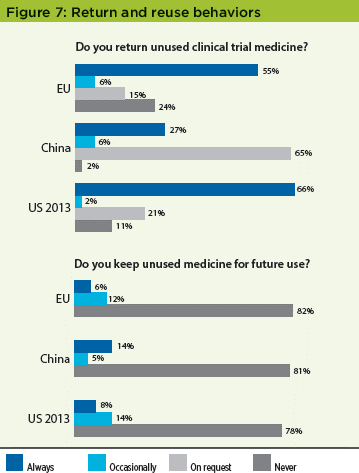
Return and reuse behaviors
The results from the EU and China were consistent with the results in the original US study, which found that a high percentage of patients did not return unused medication to the clinical sites, with ±20% of patients in all studies at least sometimes keeping the medication for future use, a result that the industry needs to mitigate against globally (Figure 7). The high "on request" results for the China study may reflect the patient’s interpretation of the question and represent those patients that returned supplies as they were "requested" to do so by the clinical site.
Home delivery
As patients often have to travel long distances to participate in studies, in order to optimize patient recruitment and retention, there is a growing interest by some sponsors to undertake clinical trials where the IMP is sent to patients’ homes. The survey team wanted to gauge patients’ future preferences for this. Over 70% of patients in both the EU and China reported that having IMPs delivered directly to their homes would be helpful; these results were very similar to those reported in the original US study (Figure 8).
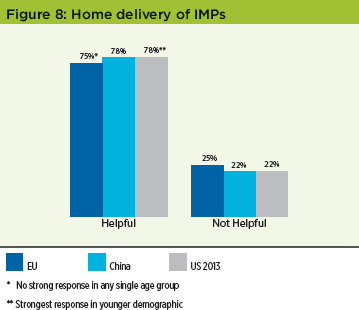
One interesting finding was that in the EU and China this preference was not significant in any single age group, whilst in the original US survey this preference was much stronger in the younger demographic. This may be a result of increased industry focus in implementing this method of delivery in the two years between the studies.4
Booklet labels
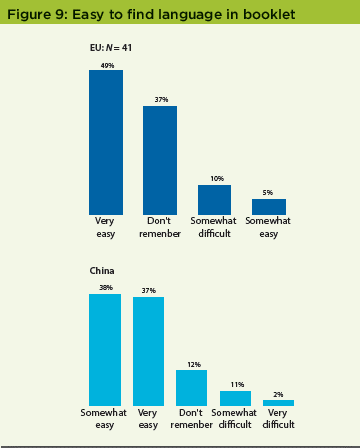
In the EU survey, patients were asked if their IMP kit had a booklet label. Less than a third—23 patients out of 80 who responded to this question (29%)—said yes, there was a label; 45% said no. Of those who saw the label, 20 (45 %) opened and read the booklet label on at least one container. Most EU patients (54%) who read the booklet label found it easy or somewhat easy to view their language, and 71% said that the text was large enough to read. In the China study, 50% of respondents did not see a booklet label on their IMP kit; 41% did. Of those who saw the booklet label, 83% opened and read it on at least one container. Of the Chinese patients who read the booklet label, 75% found it easy or somewhat easy to view their language, a larger proportion than in the EU study (Figure 9).
Other sources of information
EU patients may have received dosing information on their medicine kit from a source other than the booklet label; the largest alternative source was the receipt of verbal instructions (21%; N= 109). When patients in the China study were asked if they received instructions from a source other than the booklet label, 45% said they had, 55% said they hadn’t. Of those patients who received information from a source other than the booklet label, 59% received instructions on a patient card or leaflet (Figure 10).
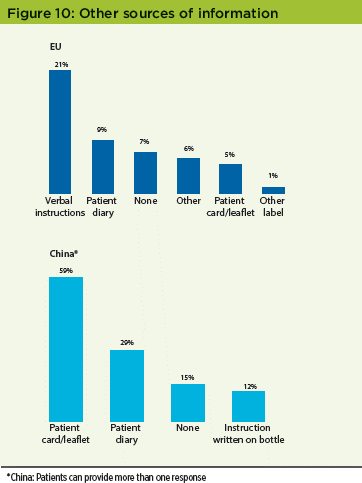
In the original 2013 US study, 34% of respondents reported seeing a booklet label on their IMP kit; 42% did not see a booklet label.
Pictograms
Since pictograms can be used in place of text that would otherwise have to be translated, the survey team wanted to gauge patients’ understanding of certain pictograms. Patients were asked to identify each picture in the pictograms below (Figure 11) from a range of provided options.

The correct answers were:
- Store between 2°C and 8°C
- Do not freeze
- Protect from moisture
- Protect from light
In the EU study, although 75% had not seen pictograms on their kits; for the examples provided, however, 96% (N = 62) identified them correctly. Patients commented that pictograms to depict storage would be the most useful; 51% of EU patients found text alone helpful, 41% found text and pictogram together helpful, with only 8% preferring pictograms alone. This unequivocal response may reflect current unfamiliarity with pictograms, and could change as the symbols are standardized and adopted more widely. In the China study, > 82% of respondents found the pictograms at least somewhat helpful.
Future information preferences
The survey team wanted to gauge patients’ preferences for the way they would like to receive information in the future. The results demonstrated that there are geographical preferences in the way that patients wish to receive information on their medication, clinical trial, or future visits. Patients in all regions indicated a strong preference for text (Table B). It is interesting to note, however, that email was the most preferred method in the EU and US, but least preferred in China, potentially because email is not significantly used as a daily or instant electronic communication tool in China.

- 4 Eli, Massimo, Catherine Hall, Marianne Oth, Adrian Peskett, and Esther Sadler-Williams. "Establishing and Managing Processes Enabling Delivery and Returns of Investigational Medicinal Products (IMPs) to Patients’ Homes." Pharmaceutical Engineering 34, no. 6 (November/December 2014).
Discussion
All of the studies—the original US study as well as the EU and China surveys—suggests an overall high level of satisfaction with IMP presentation. While current IMP kit packaging and labeling conveys a significant amount of information, however, there is no clear message that current designs improve compliance. In the EU and China studies, as many patients said that the design of the kit did not help with taking their medicine on schedule as those that said it did. This was a stronger message than in the original US study, where < 60% of patients said the design was helpful. It also suggests there is room to improve the design of the kits to improve compliance and adherence. Patient feedback to questions about what would help indicate that more attention needs to be paid to providing individual dosing units within kits where possible, as well as clear and unambiguous label information. The EU and China studies also confirmed a key finding from the original US study: Personal explanations from clinical research staff are instrumental in ensuring that patients have a positive experience, understand the study protocols, and grasp both the importance of compliance and how to achieve it. This was particularly true for patients in the China study. In view of the importance to the patient, as an industry we may need to consider controlling the way verbal information is provided to patients by the clinical site to ensure that it is provided in a consistent manner across sites and geographies.
The IP team initially expected that patients would complain most about the size, weight, and ease of transporting the kits, but they did not. Transportation and storage of the medicine to their home was not reported as an issue; in both the EU and China surveys over 70% of patients reported that their kit was the right size and over 80% found it easy or fairly easy to store at home, results that were consistent with the US findings. Kit size and weight, in fact, were deemed less important than clear label dosing information and instruction from clinical site personnel. This general satisfaction with kit size and weight may explain why patients generally did not remove drug from the medicine container. In addition, the fact that size and weight did not feature as one of the most important criteria in "kit characteristics" could be because this is a "given" to the patients that were surveyed in that they believe that the size and weight will be the smallest possible. These studies indicated that US and Chinese patients favor bottles, whilst patients in the EU prefer a blister format if possible—although in the EU and China there were still a similar number of patients who did not have a preference for either. These studies were also intended to help evaluate booklet labels, an area of intense focus in clinical trial design. Researchers in the studies wanted to see if the booklet label is an effective way to communicate medical information to patients.
This is an important issue for regulators, who are concerned that patients do not read booklet labels; a perception expressed by some is that medicine kits are often returned with unopened booklets. Although the cohort of respondents who remembered receiving booklet labels was small, 55% of patients in the EU and 17% of patients in China never opened their booklet labels. Although this showed a geographic difference, results from both regions indicated that patients frequently prefer and rely on verbal information from the clinical site rather than booklet labels. On a positive note, however, patients who did read their booklet labels found it easy to find their language and read the information; most EU patients found that the text size was large enough to read. Pictograms may be another emerging vehicle for communication, especially about storage information. In these studies, nearly all the EU patients were able to identify them correctly, and a majority of China respondents found them at least somewhat helpful. This corresponds to the original US 2013 survey, in which most patients found the same pictograms helpful. Finally, as in the original US study, these studies confirmed that nearly 20% of patients retain medicines for future use. This remains a concerning statistic in terms of potential patient safety issues; it will be important for the clinical supply community to consider strategies to mitigate against this by defining robust processes to ensure that all unused medications are returned to the clinical site. In summary, whilst many survey responses were consistent in all of the studies, some regional characteristics were apparent: e.g., medicine form preferences, packaging and reminder methodology preferences.
Conclusions
- Overall patient experience with medicine kits is very positive; patients report strong compliance and a high level of satisfaction with packaging and instructions.
- Most (> 85%) of patients found IMPs easy to use; > 76% took their medicines on schedule as planned; > 72% said that kit size and weight made the medicine easy to store and transport.
- EU and China studies show less convincing data than the US study that kit design helped patients take medication on schedule.
- In all studies, IMP kit size and weight were considered less important than clear instructions.
- Site personnel play a key role in conveying dosage information, explaining medication regimens, and ensuring that patients have positive experience and that they comply
- In the China study, 75% of patients who opened their booklet labels found it easy to find their language; in the EU study, the figure was 54%.
- Patients rely more on verbal information from clinical site personnel than on information contained in the booklet label, however.
- Technology is not frequently used to support visit and medicine reminders at present, but patients would welcome it. While regional differences among responses were apparent, text reminders were universally liked.
- EU, China, and US studies all found that ±18% of patients keep IMPs for future use; the industry should mitigate against this globally by improving clinical trial medicine return process.
- Medicine kit design and labeling could play an even stronger role in assisting compliance through the provision of clear:
- Dosing information
- Product handling information (e.g., pictograms for storage)
- As in the original US study, most patients in both the EU and China studies said that a home-delivery option for IMPs would be helpful, although unlike the original US study, there was no significant difference between age groups.
Next steps
The EU task team has concluded that it is unlikely that further valuable differentiating information will be obtained from more EU countries by translating the survey. The team are exploring a consolidated analysis of US/EU/China data, however. The teams are also supporting Japan to increase the level of patient feedback from this important region; assuming it is successful, it is hoped that the Japanese data could be included in the final consolidated analysis. Within ISPE, a task team is exploring the usefulness of pictograms for IMPs. This task team is due to provide its initial recommendations and suggestions for future study in the near future; suggestions that may include initiating dialogue with regulatory agencies on this important topic. Finally, the IP CoP is now considering how to create a form of global guidance for sponsors and clinical sites, guidance that will be critical to supporting a patient-centric supply chain of the future.