Non-GMP or GMP Washers and Sterilizers: How to Choose
This article presents the standards and guidelines typically used by manufacturers to design and build GMP washers and sterilizers. It describes the characteristics that differentiate GMP from regular laboratory equipment.
Automated washing systems and steam sterilizers (autoclaves) are often used in research and drug-manufacturing facilities to clean and sterilize a variety of items. Washers use water, cleaning agents, and mechanical action to remove residues from soiled laboratory and manufacturing-component surfaces. Sterilizers use steam to deactivate biological waste, sterilize cleaned laboratory and drug-manufacturing components, or terminally sterilize drug products.
Since a wide range of washers and sterilizers designed for various applications is available on the market today, the following questions are often raised:
- What is a GMP washer?
- How do you describe a GMP sterilizer?
- Why are GMP washers and sterilizers so much more expensive than laboratory units?
- What are the most significant differences between the two types of equipment?
- Why does it take more time to procure a GMP unit?
- When do I need to consider a GMP system instead of a non-GMP system?
- Can I turn my existing regular equipment into a GMP system?
These are all good questions!
What Are GMPs?
GMP stands for “good manufacturing practice,” a standard that is observed in regulated pharmaceutical-manufacturing facilities. GMP is also often used, rightly or wrongly, as a qualifier when describing pieces of equipment, such as the washers and steam sterilizers that are used in these facilities.
This article highlights the standards and guidelines typically used by manufacturers to design and build GMP washers and sterilizers. It describes the characteristics that differentiate GMP from regular laboratory equipment. Finally, a side-by-side comparison summarizes the main differences between the two types of equipment and describes typical applications for each.
cGMP The US Food and Drug Administration (FDA) ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with its current Good Manufacturing Practice (cGMP) regulations. These contain the minimum requirements for the methods, facilities, and controls used in the manufacturing, processing, and packing of a drug product. The regulations make sure that a product is safe for use and that it has the ingredients and strength it claims to have.1
One can see that the word “manufacturing” in GMP actually refers to the production of food, drug products, and active pharmaceutical products; it does not refer to equipment. So, the term “GMP washers and sterilizers” could be somewhat misleading. “Pharmaceutical-grade washers and sterilizers” is perhaps a more appropriate term, so it will be used throughout the rest of this article.
GMPs for pharmaceutical-grade washers and sterilizers FDA cGMP regulations for finished pharmaceuticals are provided in the Code of Federal Regulations (CFR), Title 21, parts 2102 and 211.3 Unfortunately, the regulations provide limited information concerning the way equipment used for this application should be designed and manufactured. However, four sections in subpart D of Part 211 do refer specifically to equipment:
Section 211.63—Equipment design, size, and location: “Equipment used in the manufacture, processing, packing, or holding of a drug product shall be of appropriate design, adequate size, and suitably located to facilitate operations for its intended use and for its cleaning and maintenance.”
Section 211.65—Equipment construction: “Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product.”
Section 211.67—Equipment cleaning and maintenance: This section covers mostly maintenance aspects, and does not provide much information concerning the design of the equipment itself.
Section 211.68—Automatic, mechanical, and electronic equipment: “Equipment used in the manufacture, processing, packing, or holding of a drug product shall be routinely calibrated, inspected, or checked according to a written program designed to assure proper performance. Written records of those calibration checks and inspections shall be maintained.”
In short, the FDA regulations provide general guidelines and few specific details related to the design and manufacture of equipment for the pharmaceutical industry. Therefore, manufacturers of such equipment must rely on other standards and guidelines, such as the American Society of Mechanical Engineers Bioprocessing Equipment (ASME-BPE) standard and the International Society for Pharmaceutical Engineering (ISPE) good automated manufacturing practice (GAMP®) guidelines.
The BPE standard is intended for design, materials, construction, inspection, and testing of vessels, piping, and related accessories— such as pumps, valves, and fittings—for use in the biopharmaceutical industry.5 GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems, published by ISPE, provides guidance on achieving compliant computerized systems that are fit for intended use in an efficient and effective manner.10
Other health care sterilization references, such as British Standard EN285,11 UK Department of Health HTM 201012 (now CFPP 01-01),13 and ISO 17665,14 are also commonly applied in pharmaceutical applications. These standards do contain some basic information on machine construction, performance, and testing requirements. Many pharmaceutical-grade units comply with elements of these standards.
Pharmaceutical Grade vs. Laboratory
The characteristics can be grouped into five categories:
- Manufacturer’s quality assurance program
- Mechanical design
- Process monitoring
- Control and software system
- Design, manufacturing, and qualification documentation
Manufacturer’s quality assurance program The ASME-BPE standard indicates that “the manufacturer shall implement a quality assurance program describing the systems, methods, and procedures used to control materials, drawings, specifications, fabrication, assembly techniques, and examination/ inspection used in the manufacturing of bioprocessing equipment.” 6 A third-party certification such as ISO 900115 is generally well accepted and recognized; in some cases, however, users prefer to conduct an audit of the supplier. Such a certification is not necessarily required for regular non-GMP applications.
Mechanical design The ASME-BPE 2014 Part System Design (SD) provides methods and guidelines to create a design framework, using proven practices for supporting efficient cleanability and bioburden control in bioprocessing systems.7 The overall objective is to prevent contamination of drug products due to inadequate cleaning or sterilization of surfaces that come in contact with the products during their manufacturing process. While it would be challenging to attempt to summarize the entire content of ASME-BPE in this article, some sections that relate directly to the design of washing and sterilization systems used in GMP facilities can be highlighted: SD-2.4—Fabrication: “Fabrication shall be performed in facilities where the product contact surfaces are protected from contamination.”
SD-2.4.1.1—Material of construction: “Generally, materials such as 316 and 316L, stainless steel, duplex stainless steels, and higher alloys have proven to be acceptable. … When nonmetallic materials are used (e.g., polymeric materials or adhesives), the owner/user shall specify which one of these materials shall carry a Certificate of Compliance. The conformance of material shall be explicitly stated (e.g., conforming to FDA 21 CFR 177 and USP Section <88> Class VI).”
Parts MM and PM provide additional guidelines for the selection of metallic and nonmetallic materials.
- 1US Food and Drug Administration. “Drug Applications and Current Good Manufacturing Practice (cGMP) Regulations. 17 December 2014. http://www.fda.gov/drugs/developmentapprovalprocess/manufacturing/ucm090016.htm.
- 2Code of Federal Regulations, Title 21, Part 210. “Current Good Manufacturing Practice in Manufacturinwg, Processing, Packing, or Holding of Drugs; General.” 21 August 2015. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=210.1.
- 3Code of Federal Regulations, Title 21, Part 211. “Current Good Manufacturing Practice for Finished Pharmaceuticals.” 21 August 2015. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=211.
- 5American Society of Mechanical Engineers, Bioprocess Equipment Group. ASME BPE—2014 Bioprocessing Equipment. 2014. p. ix.
- 10International Society for Pharmaceutical Engineering. GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems. February 2008. https://ispe.org/gamp-5.
- 11British Standards Institution. EN285:2006+A2:2009. “Sterilization. Steam Sterilizers. Large Sterilizers.” http://shop.bsigroup.com/ProductDetail/?pid=000000000030192330.
- 12UK Department of Health. NHS Estates. “Health Technical Memorandum 2010: Sterilisation.” 1994. http://www.isopharm.co.uk/validation-engineer/htm-2010.
- 13UK Department of Health. “Choice Framework for Local Policy and Procedures 01-01—Management and Decontamination of Surgical instruments (Medical Devices) Used in Acute Care. Part A: The Formulation of Local Policy and Choices.” http://www.his.org.uk/fi les/3113/7908/4699/CFPP_01_01_A_Management_and_decontamination_of_surgical_instruments_medical_devices_used_in_acute_care._Part_A_The_formulation_of_local_policy_and_choices.pdf.
- 14International Organization for Standardization. “ISO 17665-1:2006. Sterilization of Health Care Products—Moist Heat—Part 1: Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices.” http://www.iso.org/iso/catalogue_detail.htm?csnumber=43187.
- 6ASME BPE—2014 Bioprocessing Equipment. 2014. GR-3, p. 1.
- 15International Organization for Standardization. “ISO 9000—Quality Management.” http://www.iso.org/iso/home/standards/managementstandards/iso_9000.htm.
- 7ASME BPE—2014 Bioprocessing Equipment. 2014. SD-1, p. 17.

SD-2.4.2—Cleanability: This section describes how equipment should be designed so that all surfaces are cleanable: “Surface imperfections (e.g., crevices, gouges, obvious pits) shall be eliminated whenever feasible, horizontal product contact surfaces shall be minimized, the equipment shall be drainable and free of areas where liquids may be retained and where soil or contaminants could collect, and areas of low flow and low velocity or impact where soil or contaminants could collect. Fasteners or threads shall not be exposed to the process, steam, or cleaning fluids. Design of corners and radius shall have the maximum radius possible for ease of cleanability (minimum 3.2 mm).” (See Figure 1.)
SD-2.4.3—Drainability: “For sterility and cleaning, gravity is an effective way to facilitate drainage. To achieve gravity drainage, lines should be pitched to designated points at a specific slope.” The recommended slope varies between 0.5% and 2%, depending on the application (Figure 2).
SD-3—Process components: This section describes how piping, connections, and fittings should be designed to be hygienic.
The number of connections should be minimized; hygienic fittings should be used since threaded fittings are not recommended. Dead legs should ideally have a length/diameter ratio of less than 2 where possible. Stainless steel surfaces should be passivated, the use of blind welds should be avoided, and the design of pumps and associated connections should be hygienic (Figure 3).

Part SF—Surface finish: “Product contact surface requirements shall apply to all accessible and inaccessible areas of the systems that directly or indirectly come in contact with the designated product.” These requirements may vary from one application to another, but typically, for pharmaceutical-grade washers and sterilizers, the acceptable range varies between 20 and 30 μin Ra (0.51 and 0.76 μm).
Part MJ—Material joining: This part provides specific requirements for the joining (welding) of metallic materials. In general, welds in pharmaceutical-grade washers and sterilizers are expected to be “hygienic.”
Part PI—Process instrumentation: This section is dedicated to the definition of minimum requirements for process instrumentation in hygienic applications. In practice, the design of all instrumentation that is in contact with the washing or sterilization process has to be hygienic. Specifications for regular laboratory washing or sterilization systems typically do not include any of these mechanical requirements.
Process monitoring Section SD-5.3 of the ASME-BPE 2014 standard includes information on process-monitoring functions that are specific to washing and sterilization systems. As an example, section SD- 5.3.3.1.3 indicates that the “instrumentation and control architecture should be designed to communicate, monitor, and synchronize the clean-in-place (CIP) cycle and report CIP variables.” These variables, which are very similar in clean-out-of-place (COP) washers (also referred to as parts or components washers), include parameters such as time of exposure, temperature of wash and rinse solutions, chemical concentration by conductivity or volume, final rinse water conductivity or residual cleaning chemical concentration, water flow and pressure, and rotation of spray devices. Similar requirements for sterilizers (or autoclaves) are outlined in section SD-5.3.2. It is also mentioned that provisions for recording process parameters should be included and that “recording may be achieved by paper or 21 CFR Part 11 compliant electronic means.” EN285 and HTM2010 (CFPP) have specific process monitoring requirements for temperature and pressure in steam sterilizers.
The majority of pharmaceutical- grade washers and sterilizers are equipped with advanced process monitoring systems. New technologies now make it possible to perform online mon-itoring of total organic carbon (TOC) content in the final rinse water of CIP or COP washing systems (Figure 4). Of course, laboratory washers and sterilizers monitor critical parameters such as time, temperature, and pressure; but as an example, basic washers typically do not monitor parameters such as conductivity of final rinse water, TOC content, or rotation of spray devices.

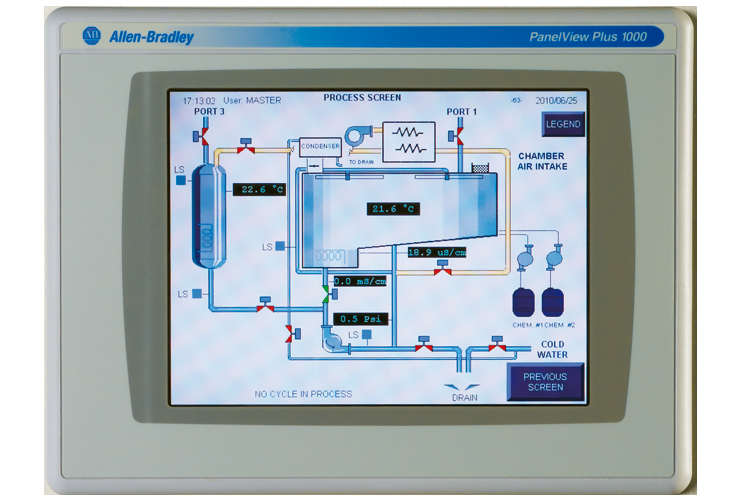
Control and software For regular laboratory applications, the type of control with which the washer or sterilizer should be equipped is rarely specified. In many cases, proprietary microprocessor-based control systems are provided and accepted. Units are typically stand-alone and rarely connected to centralized supervisory control and data acquisition (SCADA) systems. However, in GMP environments, nonproprietary commercially available control platforms are generally preferred and, in many cases, interfaced with a higher-level centralized control or data management system (Figure 5). In most cases, users expect that equipment suppliers will follow GAMP guidelines. GAMP guidance aims to achieve computerized systems that are fit for intended use and meet current regulatory requirements.8 It is also meant to provide life sciences industry suppliers with guidance on the development and maintenance of systems by following good practices. As mentioned previously, records of process parameters must be maintained for GMP applications. While printers are still commonly used, many users now opt for electronic records, in which case, CFR Title 21, Part 114 automatically applies. “This part applies to records in electronic form that are created, modified, maintained, archived, retrieved, or transmitted, under any records requirements set forth in agency regulations.”4 Again, this type of requirement does not typically apply to regular research facilities and equipment.
- 8International Society for Pharmaceutical Engineering. GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems. February 2008, p. 11.
- 4 a b Code of Federal Regulations, Title 21, Part 11. “Electronic Records; Electronic Signatures.” 21 August 2015. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=11.1.
Design, Manufacturing, and Qualification Documentation Washers and sterilizers used in research facilities are typically supplied with sufficient documentation to assist with uncrating, installation, operation, and maintenance of the equipment. General layout drawings with dimensions and information on utilities may be sent for approval prior to manufacture. Occasionally, some users may want to attend a factory approval test (FAT), which will generate the need for a dedicated FAT protocol. Good laboratory practices followed in some research facilities may dictate the need for protocols to execute installation and operation qualifications. On the other hand, regulated industries such as drug manufacturers require a lot more documentation to support the validation process. In this area, various guidelines are available and several companies have developed their own checklists. The Parenteral Drug Association (PDA) provides some guidance in Appendix B of its Technical Report 48.9 But again, BPE offers a good summary of the typical requirements in section GR-5. Not all the requirements apply to washers or sterilizers. The following is a list of those that are commonly seen:
- Complete manufacturing and certification documentation for pressure vessels: ASME, pressure equipment directive (PED), etc.
- Material test reports and heat number/code traceable to test report
- Welding documentation for pressure vessels, tanks, and piping
- Welding procedure specifications (WPSs) and procedure qualification records (PQRs)
- Welder performance qualifications (WPQs) and welding operator performance qualifications (WOPQs)
- Examiner qualifications
- Weld maps and weld logs
- Weld examination and inspection logs, coupon logs
- Purge gas certifications
- Testing and examination documentation
- Passivation reports
- Electropolishing documentation
- Surface finish report
- Spray system testing (also referred to as “coverage test”)
- Pressure testing
- Slope check documentation
- Calibration verification documentation
- Heat numbers of components must be identified, documented, and fully traceable to the installed system
- Calibration reports
- Factory acceptance test protocol and report
- Certificates of compliance for instrumentation
- Control system documentation
- User requirement specifications (URS)
- Functional requirement specifications (FRS)
- Software history
- Hardware design specifications (HDS)
- Software specifications and test reports
- Loop diagrams
- Equipment arrangement diagrams (layout drawings)
- Traceability matrix
And the list goes on …
So why are pharmaceutical-grade washers and sterilizers so much more expensive than laboratory units? The higher cost can be partly attributed to a more expensive mechanical design. Hygienic- designed components such as valves, pumps, instrumentation, sensors, and so on can be several times more expensive than their standard counterparts. The additional time required for welding and polishing to obtain the acceptable surface finish, the additional process monitoring systems, and extensive documentation also contribute to the increased cost. Another factor is that laboratory washers and sterilizers are typically standard products that can be mass-produced. Pharmaceutical-grade equipment is generally customized and made to order. This also explains why the manufacturing process for pharmaceutical-grade units is longer than that of laboratory units.
Can an existing laboratory unit be upgraded to meet GMP requirements? It is definitely possible to upgrade some components (such as the control system, piping, and instrumentation) and add some process monitoring systems. However, that is not possible for all components. Replacing a washer chamber made of stainless steel 304 with one made of stainless steel 316, for example, may prove very difficult, if not impossible. Furthermore, obtaining necessary manufacturing documentation for critical components is likely to be a challenge. Table 1 provides a condensed comparison between pharmaceutical- grade washers or sterilizers and standard laboratory units used in research facilities. It also includes a description of the typical applications for each type of equipment. Other good references include Section 3.4 and Appendix A of the PDA Technical Report (TR) 48.9 This includes design considerations of steam sterilizers.
Which One Should I Choose?
This is a common question that arises when washing and steam sterilization equipment is being selected for a facility. The most important first step is to develop a URS to define the intended use and then develop detailed design requirements according to the facility, local codes, or other requirements. Many times, units are over- or underspecified based on their intended use, and this can lead to increased costs or performance issues. A steam sterilizer used for waste disposal may not need all the features of a unit used for pharmaceutical-manufacturing area components. A laboratory washer may be able to provide the cleaning quality required without the expense of a pharmaceutical-grade unit. A pharmaceutical-grade unit can be as much as three to four times more expensive than the standard laboratory version and is more costly to validate and maintain, so having a well-defined unit is critical to the project and ongoing support cost. In general, pharmaceutical-grade equipment is required in drugmanufacturing facilities when used to clean or sterilize surfaces, parts, or components that are in contact with the drug product during its manufacturing process. For example, the following items are typically processed in pharmaceutical-grade equipment:
Change parts from drug-manufacturing filling lines such as pumps, needles, and transfer hoses
- Components from manufacturing equipment such as blenders, mixers, blister machines, tablet presses/counters, and filter housings
- Various containers, drums, and trays that come in contact with the manufacturing ingredients or the final drug product itself
- Vials, ampoules
- Process media
Laboratory-grade washers and sterilizers are generally used for research applications and decontamination of waste. Here are a few examples:
- Glass- and plasticware used in research or hospital laboratories
- Animal surgery and cage processing
- Laboratory media preparation
- Deactivation of biologically contaminated waste from laboratory research or biologic and vaccine drugmanufacturing processes.
Finally, a previously owned unit or unit procured from another area may not have the required features or cycles to meet pharmaceutical- manufacturing process needs. In this case, the upgrades, documentation, and qualification may cost more than the unit itself. PDA TR 48 provides comprehensive system design guidance in section 4.0 for steam sterilizers,9 and this methodology can be used for other types of equipment.
Conclusion
Pharmaceutical-grade washers and sterilizers used in regulated pharmaceutical-manufacturing facilities are significantly different from those used in the research industry. The requirements for these applications directly affect the mechanical design of the equipment, the process monitoring systems that need to be provided, the control system and associated software, and, most importantly, the documentation required to ensure a smooth validation process. As a result, pharmaceutical-grade systems are more expensive and take more time to procure. In general, this grade of equipment is required only if the items to be processed are in contact with the drug product that is being manufactured. It is recommended to conduct a risk assessment to help determine if a pharmaceutical-grade washer or sterilizer is really required.
- 9Parenteral Drug Association. “Technical Report 48: Moist Heat Sterilization Systems, Design, Commissioning, Qualifi cation, and Maintenance.” 2010. https://store.pda.org/TableOfContents/TR4810_TOC.pdf.
| Laboratory Washers or Sterilizers | Pharmaceutical Grade Washers or Sterilizers |
|---|---|
| Application | |
| Washing, drying and sterilization of glassware and plasticware used in laboratories, cages used for laboratory animal research |
Validatable cleaning, drying and sterilization of various materials and components used in the biotechnology and pharmaceutical manufacturing process. |
| Design | |
| Ball valves, angle body valves in stainless steel 304 or 316 or brass material | Hygienic diaphragm valves, angle body valves, ball valves and butterfly valves in stainless steel 316 material |
| Surface finish: Ra is typically not specified. | Surfaces in contact with process are typically 20 - 30 μin Ra (0.51–0.80 μm), measured and documented |
| Some blind welds may be present | No blind welds, or welds must be inspected with boroscope or other visual means |
| Some overlaps in chamber may be present | No overlaps in chamber |
| Dead legs are minimized, but not specified | Maximum 3D dead legs |
| Chamber and piping are sloped but no specific data available | Approximately 2% slopes, measured and documented |
| Standard stainless steel 304L or 316L chamber | Stainless steel 316L construction |
| Regular plastic parts | FDA approved plastics |
| No radius specified | Minimum radius of ½ inch (13 mm) |
| Regular circulation pump, stainless steel 304 or 316 (washers) | Sanitary circulation pump, stainless steel 316 (washers) |
| Regular piping in stainless steel 304 or copper with threaded, clamped or brazed fittings. |
Hygienic piping in stainless steel 316, hygienic clamp-type fittings, orbital welds, or polished welds, no threads |
| Regular instrumentation | Hygienic instrumentation |
| Single-pass rinsing system is typically not available for washers | Single-pass rinsing system generally available for washers |
| Standard filtration | HEPA or 0.2 μm filtration. Filtration may have steam-in-place and integrity test capabilities. |
| Process Monitoring | |
| Pump pressure | Pump pressure |
| Time | Time |
| Temperature | Temperature (redundant monitoring in sterilizers) |
| Detergent concentration for wash solution (conductivity or flow) | Detergent concentration for wash solution (conductivity or flow) |
| Rinse water residues (conductivity) may be available as an option for washers | Rinse water residues (conductivity) almost always provided for washers |
| N/A | TOC can be available on washers |
| N/A | Spray arm monitoring can be available on washers |
| Integral printer | Integral printer |
| Interface with SCADA system is possible | Interface with SCADA is readily available |
| Pressure in sterilizer chamber and jacket | Pressure in sterilizer chamber and jacket |
| Temperature distribution within the sterilizer chamber, including drain temperature, is guaranteed to be within ± 1.0°C (1.8°F) of the process sterilization temperature (exposure set point) |
Temperature distribution within the sterilizer chamber, including drain temperature, is guaranteed to be within ±0.5°C (±0.9°F) of the process sterilization temperature (exposure set point) |
| Control System | |
| Proprietary microprocessor-based system or commercially available programmable logic controller |
Commercially available programmable logic controller or industrial PC |
| Programmable cycles | Programmable cycles |
| Color touch screen | Larger color touch screen |
| No CFR 21, Part 11 capability | System provides capabilities to allow for compliance with CFR 21, Part 11 |
| GAMP guidelines may not be used | GAMP guidelines are followed |
| Accessories for Washers | |
| Design may not be fully hygienic, screws, welds not polished, stainless steel 304, plastic parts may not conform to FDA 21 CFR 177 and/or USP Section <88> Class VI |
Hygienic design, no screws, polished welds, stainless steel 316, plastics conform to FDA 21 CFR 177 and/or USP Section <88> Class VI |
| No manufacturing documentation provided | Manufacturing documentation provided (welding, material certificates, surface finish, etc.) |
| Documentation and Qualification | |
| Basic submittal package typically limited to technical data sheets and equipment drawings. |
Complete submittal package, typically includes equipment drawings, process & instrumentation diagram with parts list, general arrangement drawings, wiring diagrams, functional specifications, project schedule, FAT protocol |
| Uncrating, installation, operating and maintenance instructions are provided | Uncrating, installation, operating and maintenance instructions are provided |
| Manufacturing and certification documentation for pressure vessels (ASME, PED, etc.) is supplied |
Manufacturing and certification documentation for pressure vessels (ASME, PED, etc.) is supplied |
| Manufacturing documentation is typically not provided |
Complete manufacturing documentation can be provided:
|
| Control system documentation typically not provided |
Control system documentation can be provided
|
| FAT protocol and execution is possible but not typically performed | FAT protocol and execution is very common |
| Installation and operation qualification protocols and execution, site acceptance tests can be provided but this is not typical |
Installation and operation qualification protocols and execution, site acceptance tests are available |
| Note: These are typical descriptions and may vary by manufacturer. | |


