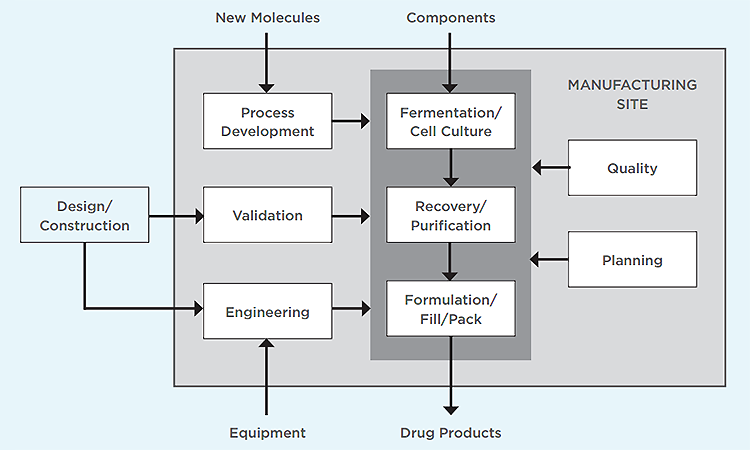
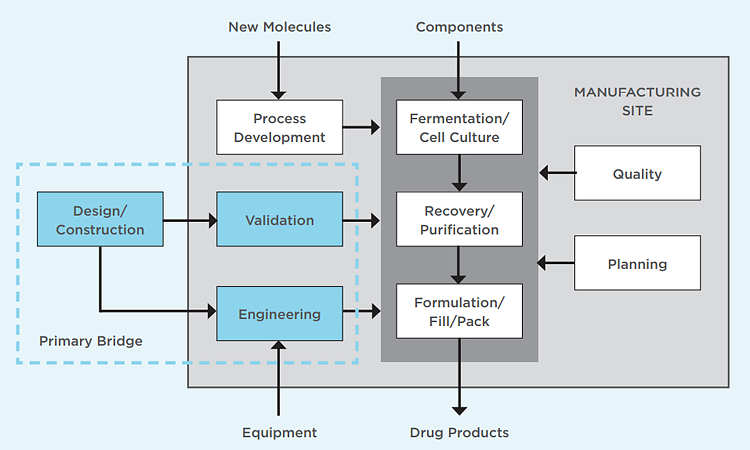
Engineers looking to enter the biomanufacturing workforce may feel unprepared without a background in cell culture or chromatography. There are, however, several positions that provide process support or technical assistance to production operations that require no direct experience with biological products. These can serve as effective bridges to the biomanufacturing space. Once there, it’s easier to move to another position within biomanufacturing after a few years of on-the-job training and experience.
This article describes some of the processes and skills required to produce large molecules, several of which can help individuals bridge gaps in work experience. Closing some of those gaps through technical training is also discussed.
TRADITIONAL VS. BIOMANUFACTURING PHARMACEUTICAL PROCESSES
For this article, traditional pharmaceuticals are considered to be small-molecule active pharmaceutical ingredients (APIs) or dosage forms containing one or more APIs that are administered orally. These dosage forms are available from pharmacies via prescription or over the counter at retail outlets. The unit operations that produce APIs and liquid forms are quite similar to some of those used in biomanufacturing, but specialized equipment for blending, granulation, and compression is required for the production of oral solid dosage forms. In general, traditional pharmaceutical processes are fairly resistant to microbial contamination and active ingredients are stable during processing.
Biomanufacturing, on the other hand, yields biological products that contain whole cells or complex proteins. These biological systems must retain their structure or post-translational modifications, or both, to maintain functionality, so they can’t be ingested and exposed to the harsh environment of the gastrointestinal tract. Instead, they are typically administered via injection or infusion, often in a clinical setting.
Critical attributes of any injectable drug product are sterility and stability. Manufacturing processes involve fermentation, mammalian cell culture, and recovery or purification operations such as centrifugation, microfiltration, column chromatography, and ultrafiltration. The requirement for drug products to be sterile and have low endotoxin levels leads to special processing to maintain low bioburden and a final aseptic filling process. Process failures can result in microbial contamination, unwanted byproducts, or both.