Balancing Pre and Post-Market Control of Health Supplements

Regulatory authorities (RAs) worldwide employ different terminologies to describe various medicinal and health products. According to the definition by the Association of Southeast Asian Nations (ASEAN), Health supplements (HS) are products used to supplement a diet as well as to maintain and improve the healthy function of human body.1 They contain one or more active ingredients such as vitamins, minerals, and fatty acids, as well as substances derived from animal, botanical, or synthetic sources. HS are presented in dosage forms such as capsules, tablets, or liquids, but do not include sterile preparations.1
Similar products are known as food supplements (FS) in the European Union (EU)2 and dietary supplements in the United States (US).3 Other terminologies include nutraceuticals, nutritional supplements, natural health products, complementary medicine, health food, and functional food. The products defined by these different terminologies are generally similar but the way they are regulated varies from country to country (Table A).12 13 14 15 16 17 18 19
Currently, the demand for HS is increasing rapidly. Global sales of HS in 2013 was estimated at US$84.5 billion, a considerable increase from $62.5 billion in 2008.4 Reasons for consumption of HS include seeking improvements in general well-being, “boosting” the immune system, filling perceived nutrient gaps in diets, and improving joint functionality. The public may have misconceptions that HS are inherently safe on the basis that many active ingredients of HS are derived from natural sources.
Current Problems
Inconsistent Quality
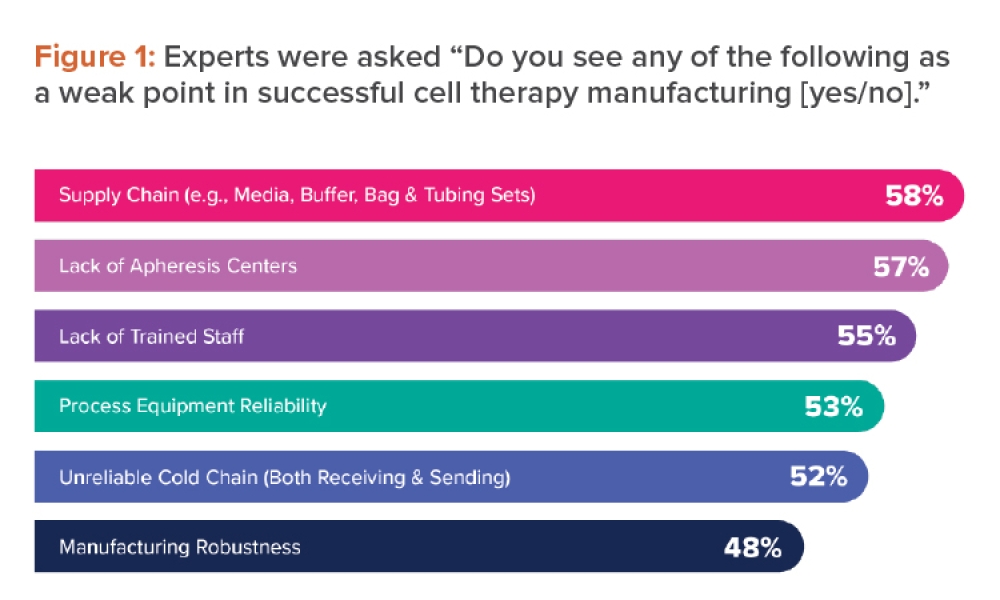
Recently, the New York State Attorney General’s Office reported that 80% of herbal HS from major global companies tested did not contain any of the herbs listed on the label. Instead, these products contained undeclared fillers such as powdered legumes and wheat.5 A review on the quality of HS sold highlighted that the actual content of active ingredients in HS products often did not match the labeled content.6 These inconsistencies and quality problems can lead to unintentional overdosing of the active ingredient(s), poisoning, and allergic reactions. Inconsistent quality is due mainly to the HS manufacturer’s poor compliance with good manufacturing practices (GMPs) and unethical practices.
Adulteration
A study was conducted on the frequency and characteristics of HS recalls in US from January 2004 to December 2012; active pharmaceutical ingredients (APIs) accounted for all of the 237 HS recalled. Most of the HS recalled were marketed as products for body-building, weight loss, or sexual performance.7 The unapproved items were usually potent APIs such as sildenafil and corticosteroids, which can cause serious consequences if taken without medical supervision. There were cases in which the consumption of adulterated HS led to severe impairment of vital organs and even death.8 Some unscrupulous manufacturers sought to avoid detection of the unauthorized ingredients by using analogues of these compounds or by incorporating these potent unauthorized ingredients in the capsule shells to avoid detection during regulatory or routine quality control tests.9
Inappropriate Claims
Most HS lack scientific evidence of efficacy. And despite guidelines established by RAs, some companies continue to market their products with inappropriate claims, such as treatment of cancer and prevention of coughs, colds, and flu.10 Both fly-by-night companies and established companies have engaged in such malpractice. These erroneous claims can pose serious health problems, as they may cause consumers to forgo prescribed medicines in favor of HS with dubious claims of efficacy.
Current Regulatory Controls
Oversight RAs may implement pre- or postmarket regulatory controls for HS, or enact a combination of both approaches. In premarket regulatory controls, the product is assessed by the RA before it is manufactured or sold. For postmarket regulatory controls, the RA may audit HS manufacturers as well as implement product surveillance programs.11 Companies are generally prohibited by most RAs from marketing their HS with medicinal claims to treat, diagnose, or prevent disease.
Harmonization and International Collaborations As mentioned in Table A, each country has its own regulatory requirements for HS. In recent years, several regions and international organizations including ASEAN (section 3.2.2) have begun to harmonize regulatory requirements to facilitate transnational movement of HS across participating countries.
Codex Alimentarius Commission The Codex Alimentarius Commission (CAC) was established by the World Health Organization (WHO) and the Food and Agriculture Organization to promote harmonization of regulatory requirements for food supplements.20 Like the EU, CAC uses the term “food supplement,” which it defines as a concentrated form of nutrient(s) used to supplement the normal diet, and is presented in dosage forms such as tablets and capsules. It has established guidelines that address the maximum and minimum daily consumption levels of vitamins and minerals, appropriate claims for such products, packaging, and appropriate choice of vitamins and minerals. The guidelines state that claims of treatment, alleviation, or prevention of disease are prohibited for FS containing vitamins and minerals.
ASEAN ASEAN is a regional organization that consists of 10 member states: Brunei, Cambodia, Indonesia, Laos, Myanmar, Malaysia, Philippines, Singapore, Thailand, and Vietnam. ASEAN is currently harmonizing HS regulatory requirements to facilitate their transnational movement among member states as part of the integrated ASEAN Economic Community formed on 31 December 2015.21 The ASEAN Traditional Medicines and Health Supplements Product Working Group is currently developing an ASEAN regulatory framework, including the harmonization of product technical requirements and GMP compliance. Already harmonized regulatory controls include the list of prohibited active ingredients, maximum limits of vitamins and minerals in HS, labeling requirements, pesticide control, additives and excipients, and the risk of transmissible spongiform encephalopathy.
- 1 a b Singapore Health Sciences Authority. “ASEAN Guidelines on Labeling Requirements for Traditional Medicines and Health Supplements.” www.hsa.gov.sg/content/dam/HSA/HPRG/Complementary_Health_Products/Annexes/ANNEX%20IX%20ASEAN%20GL%20on%20Labeling%20Requirements%20for%20TMHS.pdf.
- 2European Commission. Food Safety. Labeling and Nutrition: “Food Supplements.” http://ec.europa.eu/food/safety/labelling_nutrition/supplements/index_en.htm.
- 3US Food and Drug Administration. “Dietary Supplements.” http://www.fda.gov/Food/DietarySupplements.
- 12China Food and Drug Administration. Laws and Regulations. http://eng.sfda.gov.cn/WS03/CL0758.
- 13US Food and Drug Administration. “Dietary Supplements.” http://www.fda.gov/food/dietarysupplements/default.htm.
- 14UK Food Standards Agency. www.food.gov.uk.
- 15Singapore Health Sciences Authority. “Health Products Regulation.” www.hsa.gov.sg/content/hsa/en.html.
- 16Australia Therapeutic Goods Administration. http://www.tga.gov.au.
- 17Health Canada. www.hc-sc.gc.ca/index-eng.php.
- 18Malaysia National Pharmaceutical Regulatory Agency. “About the Drug Control Authority.” http://npra.moh.gov.my/index.php/about-npcb/drug-control-authority-dca/information.
- 19Food Safety and Standards Authority of India. www.fssai.gov.in.
- 4Feldman, Monica. “The Impact of Regulation on the Market Value of Nutraceuticals.” Presented at Discover Global Markets: Healthcare and Life Sciences Business Forum Series, 17–18 November 2015, Minneapolis, Minnesota. http://export.gov/minnesota/build/groups/public/@eg_us_mn/documents/webcontent/eg_us_mn_080867.pdf.
- 5O’Connor, Anahad. “New York Attorney General Targets Supplements at Major Retailers.” New York Times, 3 February 2015. http://well.blogs.nytimes.com/2015/02/03/new-york-attorney-general-targets-supplements-at-major-retailers/?_r=2.
- 6Lockwood, G.B. “The Quality of Commercially Available Nutraceutical Supplements and Food Sources.” Journal of Pharmacy and Pharmacology 63, no. 1 (2011): 3–10.
- 7Harel Z, et al. “The Frequency and Characteristics of Dietary Supplement Recalls in the United States.” JAMA Internal Medicine 173, no. 10 (May 2013): 926–928.
- 8Singapore Health Sciences Authority. “HSA Alerts Public to ‘OxyELITE Pro’—an Unregistered Medicinal Product Linked to Serious Liver Injuries.” www.hsa.gov.sg/content/hsa/en/News_Events/Press_Releases/2013/OxyELITE_Pro.html.
- 9Venhuis B.J., et al. “Capsule Shells Adulterated with Tadalafil.” Forensic Science International 214 nos.1–3 (2012): 20-e22.
- 10US Food and Drug Administration. “FDA Issues Warning Letters to Dietary Supplement Firms in Colorado and Texas for Promoting Unapproved Products as Drugs.” FDA News Release, 6 September 2012. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm318445.htm.
- 11US Food and Drug Administration. “Overview of the Implementation of the Current Good Manufacturing Practices for Dietary Supplements Guidance for Industry.” A prepared script for satellite broadcast, 24 October 2007. http://www.fda.gov/Food/GuidanceRegulation/CGMP/ucm173996.htm.
- 20World Health Organization. Codex Alimentarius International Food Standards. www.codexalimentarius.org.
- 21Association of Southeast Asian Nations. “Harmonising Regulation of Traditional Medicines and Health Supplements Ahead of ASEAN Integration.” http://asean.org/harmonising-regulation-of-traditional-medicines-and-health-supplements-ahead-of-asean-integration.

EU The EU implemented the Directive 2002/46/EC, approximation of the laws of the member states relating to food supplements, with the goal of facilitating transnational movement of FS among EU Member States.22 Currently, these regulations only apply to FS containing vitamins and minerals. FS containing ingredients other than vitamins and minerals will be regulated by the legislature of individual member states. The FS company has to apply for authorization from European Commission (EC) of the EU if the products contain vitamins or minerals that are not present in the list or if they contain any novel ingredients; these are ingredients that have no history of significant use before 1997. If, however, the company is able to demonstrate that the novel ingredient is significantly equivalent to an existing ingredient, the authorization process can be accelerated.
All FS sold in the EU can only contain claims approved by the EC, and claims relating to disease risk reduction will require higher level of scientific evidence. Companies are also prohibited from marketing their FS with any claims of treating, diagnosing, or preventing diseases.22 Another regulation, Directive 2004/24/EC, covers traditional herbal medicinal products, which in Europe, are generally regulated as medicines.
Effect of Differing Regulatory Frameworks There is no worldwide agreement on the definition of HS, and the differing regulatory controls across countries have proven to be a challenge for companies marketing their HS globally. As shown in Table A, HS have been regulated as food, medicinal products (MP), or “intermediate” products that straddle both categories. Product categorization helps determine the level of regulatory controls required; regulatory controls for food are usually less demanding than those for MP. The regulatory framework for HS may differ from country to country. For example, Malaysia Drug Control Authority and Health Canada have more stringent regulatory frameworks: Premarket approval is required before sale or manufacture of such products can occur. However, many RAs categorize HS as food products; these HS are regulated under food product laws. Some countries such as China and India have yet to establish a legal definition for HS. These countries categorize HS under the broad category of “health food.”23
Despite the legal definitions of HS in many countries, specific GMP standard for their manufacture have yet to be established. The UK Food Standards Agency, for instance, requires manufacturers to comply with GMP meant for manufacture of food.24 Some countries also have different requirements regarding substantiation of claims used by companies to market HS. For example, the Japanese RA requires clinical trials to be conducted for any claims made by the company before these HS can be marketed. On the other hand, animal tests are sufficient for certain HS claims in China. To further complicate matters, there have been claims for HS that are accepted by EC but rejected by US Food and Drug Administration (FDA).
Recommendations
Premarket Ingredient Approval A regulatory framework incorporating a premarket approval process can help avert situations such as the one involving ephedra-containing dietary supplements in the US about 20 years ago. Between 1995 and 1997, US FDA received over 900 reports of adverse events due to ephedra-related toxicity.25 Other botanical ingredients such as kava-kava and Yohimbe have been reported to cause liver failure and cardiovascular disorder respectively.24 These adverse events could have been averted with premarket assessments.
Over the years, EU Member States have implemented premarket regulatory controls for HS with the underlying principle that HS must be proven safe for human consumption before they can be sold. On the other hand, some HS companies opine that the EU regulatory system is too stringent and have raised this issue to the European Court of Justice. It was ruled, however, that such a regime is necessary to ensure consumers’ safety. Countries such as Canada and Malaysia have recognized the advantages of pre-market regulatory controls and have adopted similar regulations. In contrast, premarket regulatory controls for such products are minimal in the US, where the products are presumed to be safe unless proven otherwise with consumers’ access taking center stage.26
There have been counterarguments lamenting the lack of protection of consumers’ safety under the current regulatory framework in the US. In recent years, US FDA has recognized the lack of consumer protection and is reconsidering the current regulatory controls for novel ingredients in such products.27 The premarket approval system adopted by EU is a feasible form of premarket regulatory control to safeguard consumers of HS. RAs could issue a positive list of ingredients that HS are allowed to contain, and companies would be required to submit the composition of the products for approval before they could be sold. If the HS contain active ingredients not present in the list, the ingredients would be subject to safety assessments before the products could be sold. Instead of subjecting every product to individual assessment, premarket approval by ingredients is more practical. Such a regulatory system, based on a positive list of ingredients, would require fewer resources, and would hopefully result in a smaller backlog. In China, where the FDA subjects each product to premarket approval, it is estimated that it can take up to 5 years for a new product to be registered in the country.23
In comparison, it takes an average of 3 years to approve novel HS ingredients in the EU. As for HS that contain ingredients that are not in the positive list, approving them on the basis of historical use may be unwise. Historical use may not be a good indicator of safety, as evinced by the adverse events associated with supplements containing kava-kava and ephedra, which have a long history of use as “traditional medicines.” The US Committee on the Framework for Evaluating the Safety of Dietary Supplements also highlighted that historical use may not always be relevant in assessing the safety of ingredients due to issues such as differences in indications and dosage forms.28 Prohibiting the sale of these products can severely impact consumers who need them.
Two possible approaches may be adopted to avoid excessive inconvenience to consumers: The first would require affected companies to engage in safety testing akin to Phase IV clinical trials of new drugs. The companies would be allowed to sell their products while necessary safety tests are being conducted. The products would be withdrawn only when found unsafe. The second approach would allow the restricted sale of products to consumers, subject to assessment and verification by physicians, similar to the system for supply of prescription-only medicines. Both approaches, however, would imply that there would still be consumption of products despite the incomplete safety assessment of the ingredients.
As safety is very important, neither approach may be acceptable. To avoid adverse effects, the sale of potentially harmful products should be prohibited during the safety evaluation period. consumer safety should always take precedence over accessibility and business considerations. Moreover, these are not lifesaving products, and the lack of such products is not life-threatening. Therefore, if RAs were to implement a form of premarket framework for HS, the approval of any HS product or ingredient should not be based just on historical use. It has been argued that premarket regulations may reduce consumers’ access to HS by raising economic barriers to market entry. However, it is a minor drawback compared to the risks from the consumption of potentially harmful HS. A premarket regulatory system would also improve the ability of RAs to detect adulterated HS before they are sold, and to deter companies from producing such products.
Premarket Claims Approval Inappropriate claims of HS made by companies are a major problem. RAs in countries such as China and Malaysia require companies to submit claims for approval before the HS can be sold; in contrast, this is not required in countries such as the US and Singapore. The onus is on the HS industry to act responsibly and avoid making inappropriate claims. There are unethical companies, however, that will seek to boost sales by making inappropriate claims. These companies may abuse the lack of oversight by making claims that appear legal and deceive consumers into believing the HS products can treat or prevent disease26 27 28 29 . Premarket interventions can prevent inappropriate claims as the RAs would be able to reject such claims before granting market authorization.
An ideal premarket regulatory regime should require all companies to prove HS efficacy via randomized clinical trials (RCTs), similar to the premarket regulatory framework for pharmaceutical products. This would allow only products with proven efficacy to reach the market. However, RCTs can be costly, and HS companies may not have the economic resources as there are no avenues to patent their products. Additionally, it may not be practical to expect RCTs to be conducted for all types of claims. Instead, it would be more prudent for the various HS claims to be substantiated by appropriate levels of evidence, based on the nature of the HS and their risk profiles.
Establishing GMP GMP-compliant companies are better able to assure HS quality and safety as they have consistent manufacturing processes and cross-contamination prevention programs in place. Currently, there is a lack of national and international GMP standards specifically for manufacture of HS. It is essential to establish internationally acceptable GMP standards to avoid quality issues. The manufacture of HS requires appropriate process controls, suitable equipment, specialized skills, and knowledge. The occurrence of several quality-related incidents convinced US FDA to establish GMP specifically for the manufacture of HS. The US FDA also noted that because HS, unlike food products, were packaged into dosage forms such as tablets and capsules, it would be inappropriate for HS manufacturers to adopt GMP established for food manufacturers. Although GMP compliance will increase manufacturing costs, it will help improve consumers’ confidence in HS.
In the long run, this will benefit the HS industry, the RA, and the consumers. Upon implementation of the GMP inspection standard, there should be periodic audits by RAs to ensure continued GMP compliance by the manufacturers. Periodic audits are necessary as HS companies were still found to be noncompliant to GMP during ad hoc audits conducted by the US FDA despite the establishment of GMP standard for HS manufacturers.30 The 2003 Pan Pharmaceutical debacle in Australia in is a classic example of the importance of regular GMP audits. RAs can also explore other options beside implementing regulatory controls to address quality problems. These include encouraging HS companies to undergo voluntary third-party (independent) GMP certification, coordinated by industry associations.
Currently, reputable third-party organizations such as the US Pharmacopeia (USP) and NSF International conduct quality testing of products as well as GMP inspection of manufacturing facilities.31 32 HS products that bear these organizations’ stamps indicate that the manufacturers are GMP compliant. These voluntary independent certifications may provide a form of quality assurance to HS consumers.
RAs can encourage companies to undergo such certification by educating the public and health care professionals about the important role such organizations play. They could also provide guidance to assist HS companies in selecting the correct organizations33 and to single out products that are legally misbranded, or do not comply with the certifying organization’s specifications.34 These certifications could also help ease the strain on the limited resources by complementing the RA efforts in market surveillance and GMP audits, especially for overseas HS manufacturers. Organizations such as USP have established offices in countries such as India and China to facilitate the certification of HS manufacturers in these countries. RAs can also help encourage some form of industry self-regulation through formation of associations such as the Health Supplements Industry Association of Singapore, Food Supplements Europe, and the Council for Responsible Nutrition in the United States. The associations could establish their own code of ethics and mutual agreements on GMP and quality standards, thus increasing the likelihood that companies will be GMP compliant.
In some cases, the associations have established their own GMP standard specifically for the manufacture of HS despite the lack of a regulatory GMP standard. Overall, industry initiatives may complement the efforts of RAs to safeguard public health and help to ease the strain on the limited resources of RAs.
Harmonization of Regulatory Requirements Most regulatory standards for HS are currently not harmonized. In some countries such as China and Australia, HS are required to be registered with the RAs while in many other countries, premarket licensing or approvals are not mandatory. This leads to a diverse range of regulatory requirements for the HS industry to manage. Harmonization of HS regulatory requirements could enhance consistency, efficiency, and cost-effectiveness for both RAs and the industry. It would facilitate cooperation and collaboration among different RAs in a highly globalized world with interconnected trade. Sharing information among different RAs can facilitate the detection of adulterated or poor quality HS and help avoid public health crises.
Harmonization is also beneficial for companies as it can reduce number and amount of regulatory submissions required and hence, reduce registration time and regulatory cost.35 It has been shown that harmonized requirements and the resulting reduced cost of product registration have encouraged pharmaceutical companies to invest more in research. The same outcome may apply to the HS industry if the regulatory requirements for HS are harmonized. However, harmonization may create economic issues for countries that have insufficient resources or expertise. Moreover, the large variation of HS products and formulations in different countries may compound the difficulty of achieving harmonization. Nevertheless, harmonization should be pursued.35 International organizations such as the WHO and the Food and Agriculture Organization of the United Nations as well as countries with relevant expertise can help countries that are lacking, through training of the regulators. ASEAN member states have been harmonizing their regulatory requirements for HS and collaborating actively with one another. Despite differences in cultural, political and socioeconomic background among ASEAN member states, significant progress has been achieved, indicating that harmonization of HS regulatory requirements at the regional or international level is possible.
Conclusion
The demand for HS is expected to grow considerably, attracting many companies to have a slice of the growing market. It is still largely a caveat emptor (buyer beware) market where tighter worldwide regulatory control for HS appears necessary. RAs should implement appropriate HS regulatory schemes to safeguard public health, but without severely affecting the consumers’ access to affordable products. As all RAs have limited resources in terms of manpower and funds, an appropriate risk-based regulatory scheme is critical. By: Chan Lai Wah, Benjamin Tan Zhi Yang, Vimal Sachdeva, and Sia Chong Hock
- 22 a b Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Official Journal of the European Union L. 183, 12 July 2002. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:183:0051:0057:EN:PDF.
- 23 a b US-China Health Products Association. “China’s Dietary Supplement Sector and Key Issues.” 3 April 2014. http://uschinahpa.org/wp-content/uploads/2012/01/China%E2%80%99s-Dietary-Supplement-Sector-and-Key-Issues-2014.pdf.
- 24 a b US Government Accountability Office. Dietary Supplements: FDA Should Take Further Actions to Improve Oversight and Consumer Understanding. GAO-09-250. January 2009. www.gao.gov/assets/290/285372.pdf.
- 25National Center for Complementary and Alternative Medicine. “Ephedra.” http://nccam.nih.gov/health/ephedra.
- 26 a b Azizi Rahi. “Supplementing the DSHEA: Congress Must Invest the FDA with Greater Regulatory Authority over Nutraceutical Manufacturers by Amending the Dietary Supplement Health and Education Act.” California Law Review 98, no. 2 (30 April 2010): 439–479.
- 27 a b Cohen P.A. “Assessing Supplement Safety—The FDA’s Controversial Proposal.” New England Journal of Medicine 366, no. 5(2012): 389–391.
- 28 a b Institute of Medicine, and National Research Council of the National Academies. Dietary Supplements: A Framework for Evaluating Safety. Washington, DC: The National Academies Press, 2004.
- 29
- 30Whitsitt V., et al. “The Role of Good Manufacturing Practices for Preventing Dietary Supplement Adulteration.” Analytical and Bioanalytical Chemistry 405, no. 13 (2013): 4,353–4,358.
- 31NSF International. “Dietary Supplement Safety.” http://www.nsf.org/services/by-industry/dietary-supplements/supplement-safety.
- 32US Pharmacopeial Convention. “USP Verified Dietary Supplements.” http://www.usp.org/usp-verification-services/usp-verified-dietary-supplements.
- 33US Food and Drug Administration. “Voluntary Third-Party Certification Programs for Foods and Feeds.” Application Process. www.fda.gov/RegulatoryInformation/Guidances/ucm125431.htm#IVA.
- 34US Pharmacopeial Convention. “USP Comments on FDA’s New Dietary Ingredient Draft Guidance.” http://www.usp.org/node/2682
- 35 a b Riviere J.E., and G.J. Buckley, eds. Ensuring Safe Foods and Medical Products through Stronger Regulatory Systems Abroad. Appendix H: “Strengthening Core Elements of Regulatory Systems in Developing Countries: Identifying Priorities and an Appropriate Role for the US Food and Drug Administration.” Washington, DC: National Academies Press, 2012. www.ncbi.nlm.nih.gov/books/NBK201164.