Is a Globally Harmonized Quality Overall Summary Possible?
Cover: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline on Registration of Pharmaceuticals for Human Use (M4) offers advantages in the consistent format of the registration dossier using the Common Technical Document (CTD). However, it does not deliver a comprehensive view of the overall manufacturing control strategy or a means of understanding and managing the quality of the product throughout its life cycle. As a result, several regulatory authorities that have implemented the CTD format have also insisted on supplementary quality summary documentation that exceeds ICH requirements, and, in effect, creates divergent expectations for chemistry, manufacturing, and controls (CMC) content. A single global quality overall summary (QOS) format could clearly convey a holistic view of a product’s control strategy and improve the efficiency and economy of the regulatory review of an application while providing a way for the applicant and reviewer to align on a product life-cycle management plan.
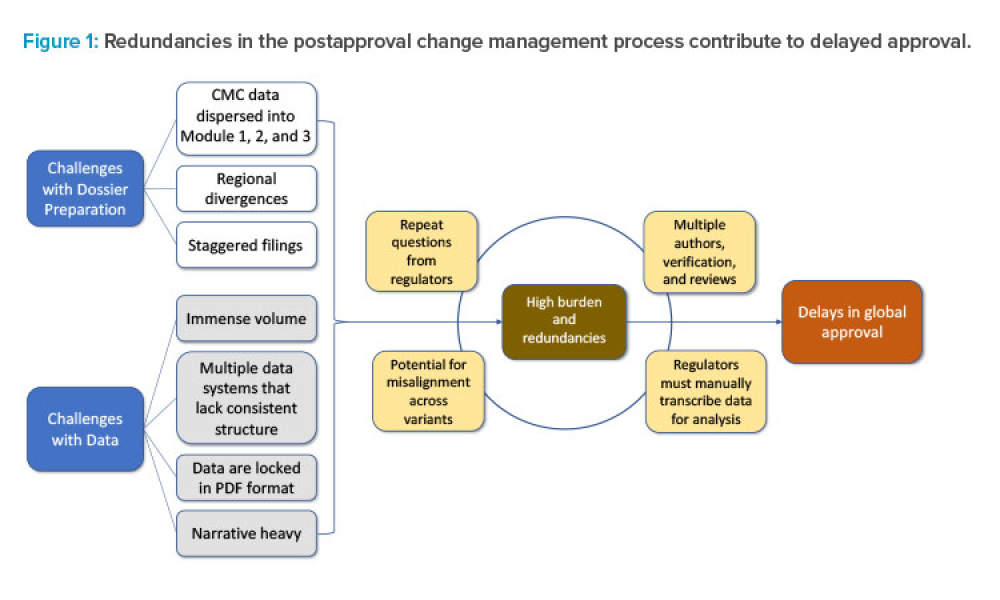
Streamlining Postapproval Submissions Using ICH Q12 & SCDM
Feature: Postapproval change management of pharmaceuticals is an essential part of life-cycle management but is associated with regulatory challenges. Incorporating concepts and tools from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline, combined with structured content and data management (SCDM) and a cloud-based data exchange platform, could provide synergistic benefits that will enable efficient supply maintenance of life-saving therapies worldwide.
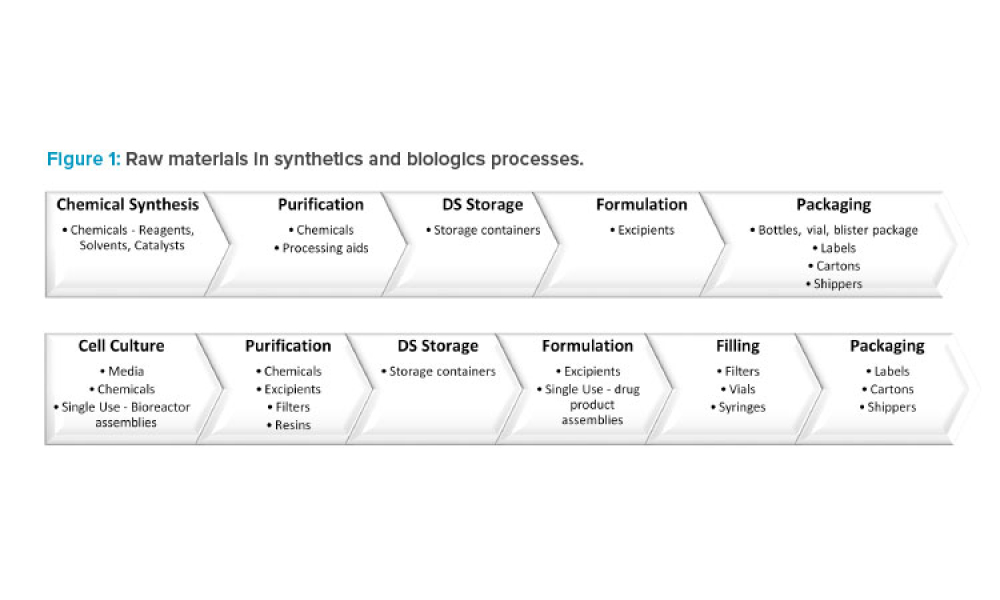
Regulatory Landscape for Raw Materials: CMC Considerations
Feature: A reliable supply of raw materials is critical to maintain a robust supply chain to serve patients globally. With shortages, regulatory complexity is compounded due to differences in submission and data requirements from various regulatory agencies. Therefore, there is an increasing need to implement a harmonized regulatory infrastructure that is both flexible and predictable to provide more agility without product delays.
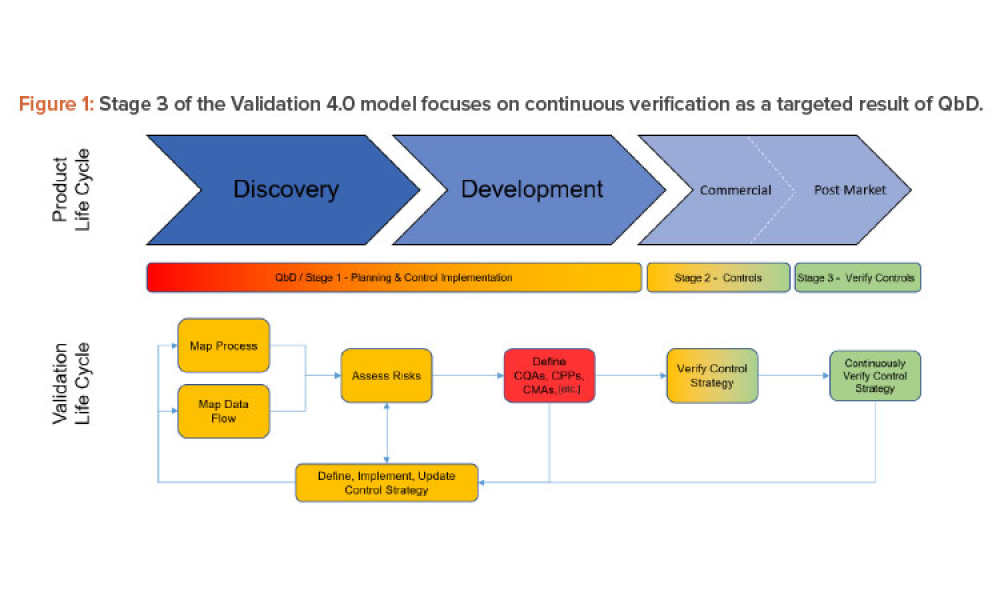
Validation 4.0: Case Studies for Oral Solid Dose Manufacturing
Technical: Three case studies on Validation 4.0 demonstrate how quality by design (QbD) principles, when applied with digitization, can verify processes in scale-up and technology transfer, and why blend and content uniformity matter for tablet integrity.
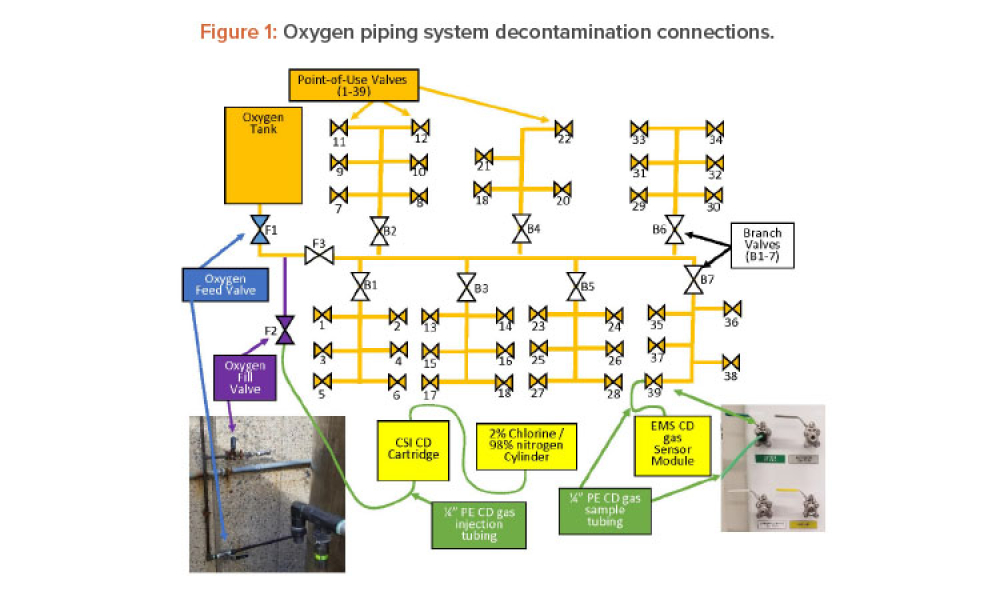
Novel Dry Decontamination Method Using: Gaseous Chlorine Dioxide
Technical: Chlorine dioxide has been shown effective in decontaminating various types of chambers and volumes such as rooms, isolators, processing tanks, and entire facilities, but its use to decontaminate compressed gas piping systems has not been documented. This article discusses using dry gaseous chlorine dioxide (ClO2) to decontaminate an oxygen (O2) feed piping system in a pharmaceutical research laboratory and shows that a dry gas can be used to remediate a contaminated piping system.